Preparation and application of anti-human programmed death factor 1 (PD-1) monoclonal antibody
A monoclonal antibody, PD-1 technology, applied in the field of biomedicine, can solve problems such as structural differences and deficiencies, and achieve high affinity and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Stable membrane expression of hPD-1.
[0026]In order to obtain a cell line expressing hPD-1 protein on the cell membrane surface as an immunogen, a cDNA clone encoding the full-length open reading frame of human PD-1 was purchased (Beijing Sino Biological Technology Co., Ltd. HG10377-CF, Genbank accession number NM-005018.2) , insert it into the pCDNA3.1 (+) (Invitrogen Company) vector, and sequence it to confirm that the coding frame of hPD-1 gene is correct. The plasmid was electrotransformed into CHO-DG44 cells, pressurized screening, and cloned to obtain CHO-DG44 cells stably expressing hPD-1. Named hPD-1 / DG44.
Embodiment 2
[0028] Animals are immune.
[0029] Five A / J mice aged 6-8 weeks were selected and immunized five times in total, with an interval of 14 days between each immunization. 10 per immunity 7 hPD-1 / DG44 cells, subcutaneous and intraperitoneal multipoint immunization. For the first immunization, an equal volume of Freund's complete adjuvant was mixed with cell suspension, and for the remaining 4 immunizations, Freund's incomplete adjuvant was mixed with cell suspension.
[0030] Cell culture medium configuration.
[0031] MD6 serum-free medium was used for culturing sp2 / 0 cells. After fusion, the cells were cultured in HAT medium, and the specific components were as follows: 10% fetal bovine serum and HAT were added to MD6 serum-free medium.
[0032] Cell fusion.
[0033] Three days after the final immunization, three mice with high titers were selected for cell fusion. Aseptically remove the mouse spleen and the pre-prepared sp2 / 0 cells, count the cells, mix them at a ratio o...
example 3
[0041] Functional characterization of anti-hPD1 monoclonal antibody.
[0042] Affinity identification.
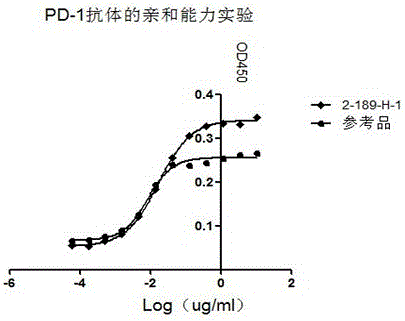
[0043] The 96-well ELISA plate was coated with hPD-L1-Fc (RD); the blocking antibody was diluted 3 times as the primary antibody, and a total of 12 gradients were added to the 96-well ELISA plate coated with hPD-L1-Fc. Add goat anti-mouse IgG-Fc-HRP (SANT CruzBIotechnology) as the secondary antibody, add the chromogenic solution, and read the OD450 value after termination. EC50 concentrations were generated using Graphpad software. The EC50 of this antibody is 1.0667nM, and the EC50 of the positive control is 0.5344nM. It can be seen from the results that the screened antibody has obvious affinity for PD-1 and is similar to the positive control (see figure 1 ).
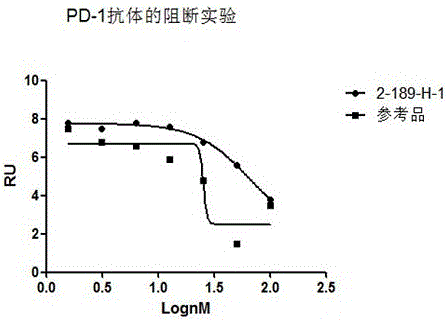
[0044] Functional characterization of antibodies blocking hPD-1 binding to hPD-L1.
[0045] A 96-well ELISA plate was coated with hPD-L1-Fc (RD); the purified hPD-1 antibody was diluted 4 times, and a total of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com