Determination method and application of biological activity of recombinant human epidermal growth factor
A technology for epidermal growth factor and biological activity, applied in the field of biological activity determination of recombinant human epidermal growth factor, can solve the problems of cumbersome biological determination method, long experimental period, poor accuracy, etc., and achieves convenient implementation and high accuracy , Quality controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
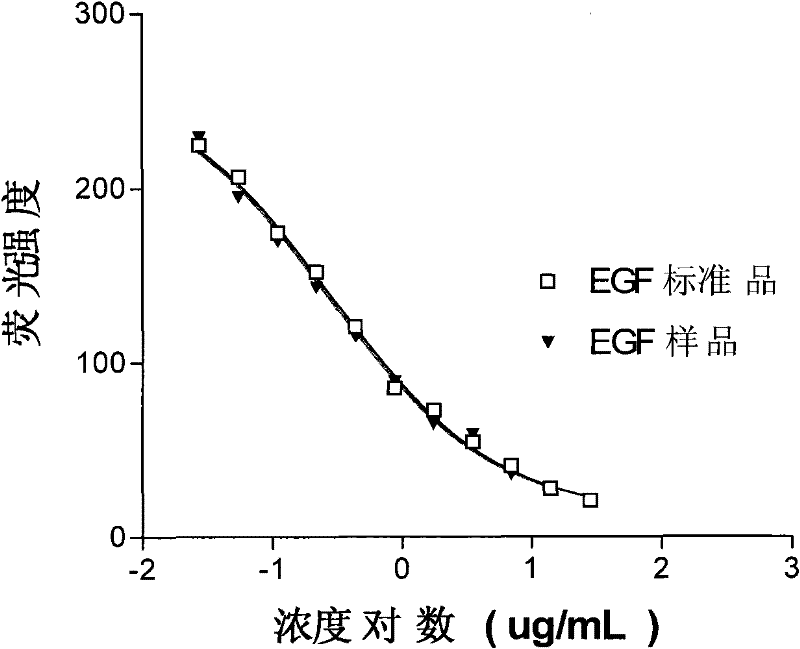
[0035] Example 1 Recombinant human epidermal growth factor activity determination method
[0036] The method of measuring EGF activity using human lung adenocarcinoma cell line H-125 (from Cuban Molecular Immunology Center) includes the following steps:
[0037] (1) The cell culture laboratory cultures a certain amount of H-125 cells, and the cell viability should generally not be less than 70%. Centrifuge at 4°C and 1100 rpm for 5 minutes. Carefully discard the supernatant, use the remaining small amount of liquid to dissolve the cell pellet, and then slowly add about 10 mL of PBS and mix well. Centrifuge again at 4°C and 1100 rpm for 5 minutes, carefully discard the supernatant, use the remaining small amount of liquid to dissolve the cell pellet, slowly add about 10 mL of PBS, and mix. After centrifugation at 4°C and 1100rpm for 5 minutes, carefully discard the supernatant, use the remaining small amount of liquid to dissolve the cell pellet, adjust the cell suspension of the ...
Embodiment 2
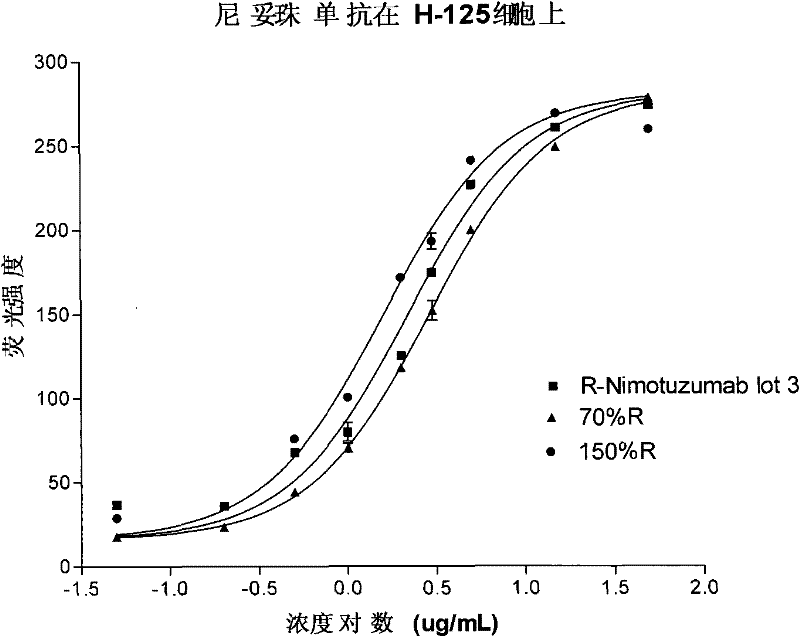
[0049] Example 2 The reproducibility of the biological activity of epidermal growth factor measured by flow cytometry
[0050] Methodological validation of the relative biological binding activity of Nimotuzumab using the same flow cytometry method:
[0051] The method for measuring the relative biological activity of Nimotuzumab using the human lung adenocarcinoma cell line H-125 (from the Cuban Molecular Immunology Center) includes the following steps:
[0052] (1) Prepare 50μg / mL nimotuzumab reference diluent and sample diluent, then dilute 8 dilutions with monoclonal antibody diluent to 0.050μg / mL;
[0053] (2) Add 20 μL of reference diluent and sample diluent of different concentrations to each centrifuge tube, and the subsequent steps are the same as in Example 1;
[0054] (3) Data processing: computer program (four-parameter regression) is used for processing, and then the half effective binding concentration (EC) of the nimotuzumab reference product and sample 50 ) To obtain the...
Embodiment 3
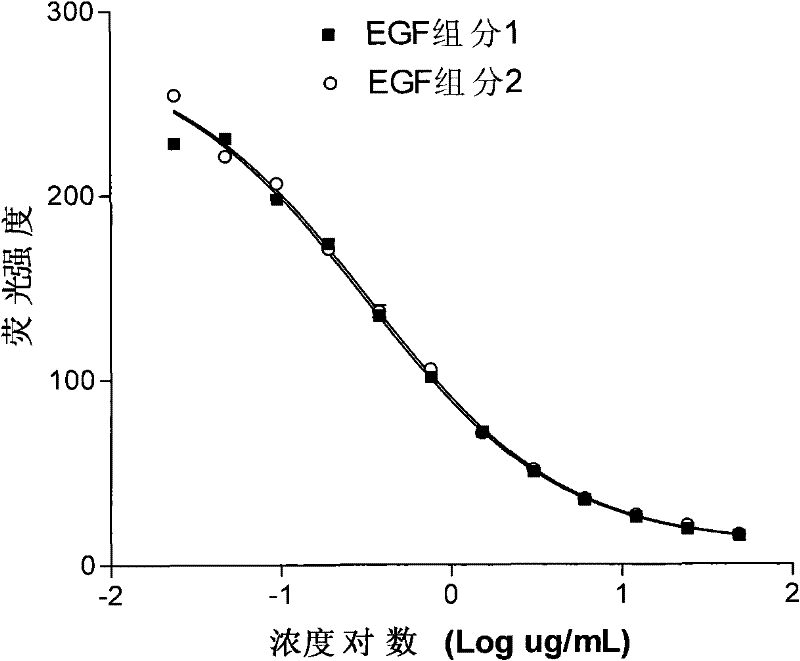
[0086] Example 3 Comparison of biological activities of different recombinant human epidermal growth factors
[0087] EGF component 1 is the C-terminal deletion of arginine and leucine of human EGF (EGF of 51 amino acid residues)
[0088] EGF component 2 is the C-terminal deletion of arginine of human EGF (EGF of 52 amino acid residues)
[0089] The biological activity of EGF was determined according to the steps of Example 1. The test data are shown in Table 6.
[0090] Table 6 Comparison of biological activities of different rEGF
[0091] EGF component 1
EGF component 2
Minimum
10.00
10.00
Max
279.1
279.1
LogED 50
-0.5096
-0.4883
Slope (Hillslope)
-0.7530
-0.7530
ED 50 (ug / mL)
0.3093
0.3249
Curve Fit R 2
0.9987
0.9976
[0092] the result shows:
[0093] ED 50 (EGF component 1)=0.3093ug / mL
[0094] ED 50 (EGF component 2) = 0.3249ug / mL
[0095] The biological activity of EGF component 2 relative to EGF component 1 is 95.2% (EC 50 It is inversely ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com