Synthesis and application of RGD polypeptide coupled SiPc (silicon phthalocyanine) photosensitizer
A technology of silicon photosensitizer and silicon phthalocyanine, which is applied in the field of new photosensitizers for photodynamic therapy of tumors, can solve problems such as failure to meet performance requirements and weak photoactivity of zinc phthalocyanine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
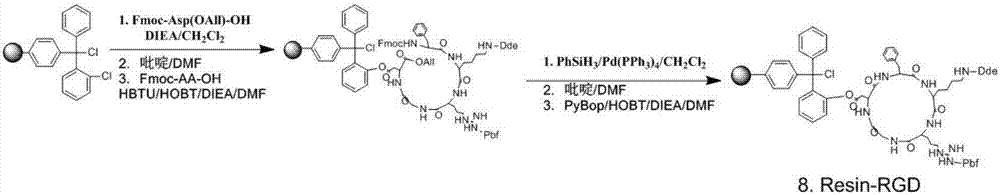
[0030] Synthesis of embodiment 1 RGD polypeptide (attached figure 1 )
[0031] We first synthesized RGD polypeptide compounds by solid-phase method. The resin was swollen with dichloromethane 1 h before the synthesis, and we used a triphenyl chloride resin with a loading of 0.5 mmol / g. Dissolve Fmoc-Asp(OAll)-OH (1.0 g, 1.0 mmol) and N,N-diisopropylethylamine (DIEA) (680 μL, 4.0 mmol) in 10 mL N,N-dimethylformamide (DMF ) into the solid-phase synthesizer, and reacted at room temperature for 5h. Then configure the blocking solution (dichloromethane CH 2 Cl 2 : Methanol MeOH: DIEA=17:1:2), added to the solid-phase synthesizer to block unreacted chlorine. The Fmoc protecting group was removed with 20% piperidine in DMF for 30 min. The coupling reaction of four amino acids (Fmoc-Gly-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Lys(Dde)-OH and Fmoc-D-Phe-OH) was protected by 1.5eq. of Fmoc in each step In-situ addition of condensing agent O-benzotriazole-tetramethyluronium hexafluorophosphate...
Embodiment 2
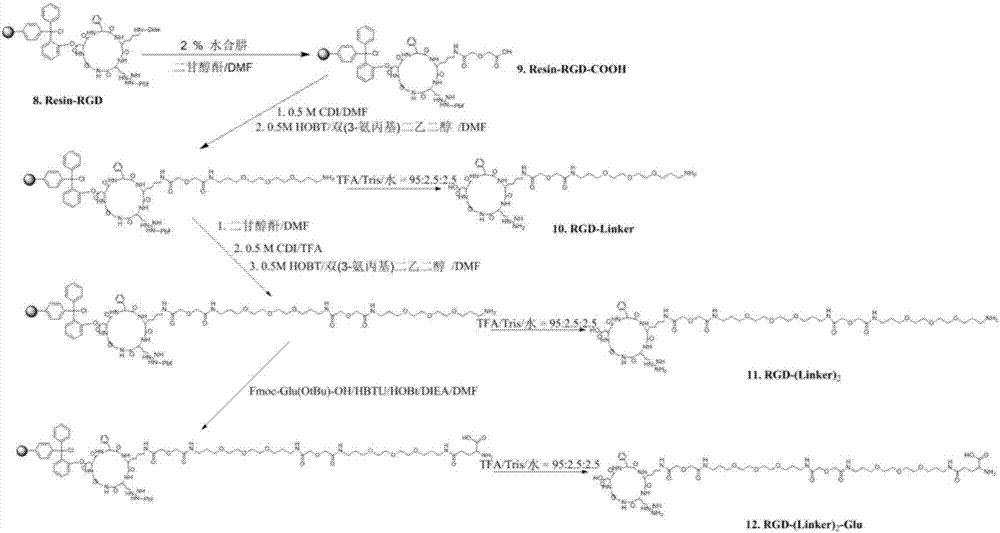
[0033] Embodiment 2 RGD-Linker, RGD-(Linker) 2 and RGD-(Linker) 2 -Synthesis of Glu (attached figure 2 )
[0034] 1. Synthesis of the compound called RGD-Linker for short
[0035] Deprotected resin-RGD (1.0g, 0.5mmol) and diglycolic anhydride (116.1mg, 1.0mmol) were dissolved in 5mL DMF and shaken for 5h to obtain the product resin-COOH. Then, resin-COOH was activated with N,N'-carbonyldiimidazole (0.5M CDI) and reacted for 1h. Activated resin-COOH, 0.5M 1-hydroxybenzotriazole (HOBt), bis(3-aminopropyl)diethylene glycol (1.1g, 5mmol) were mixed and shaken for 5h, and then mixed with DMF and dichloromethane After washing, vacuum-dry to obtain the product resin-RGD-Linker. Add 500 μL of eluent (TFA / triisopropylsilane Tris / water=95:2.5:2.5) for elution at room temperature for 1 h. Anhydrous ether (1.5 mL) was added to the eluate for precipitation, washed three times and dried at room temperature to obtain the product RGD-Linker (0.18 g, yield 39%). (HRMS-ESI: m / z calculat...
Embodiment 3
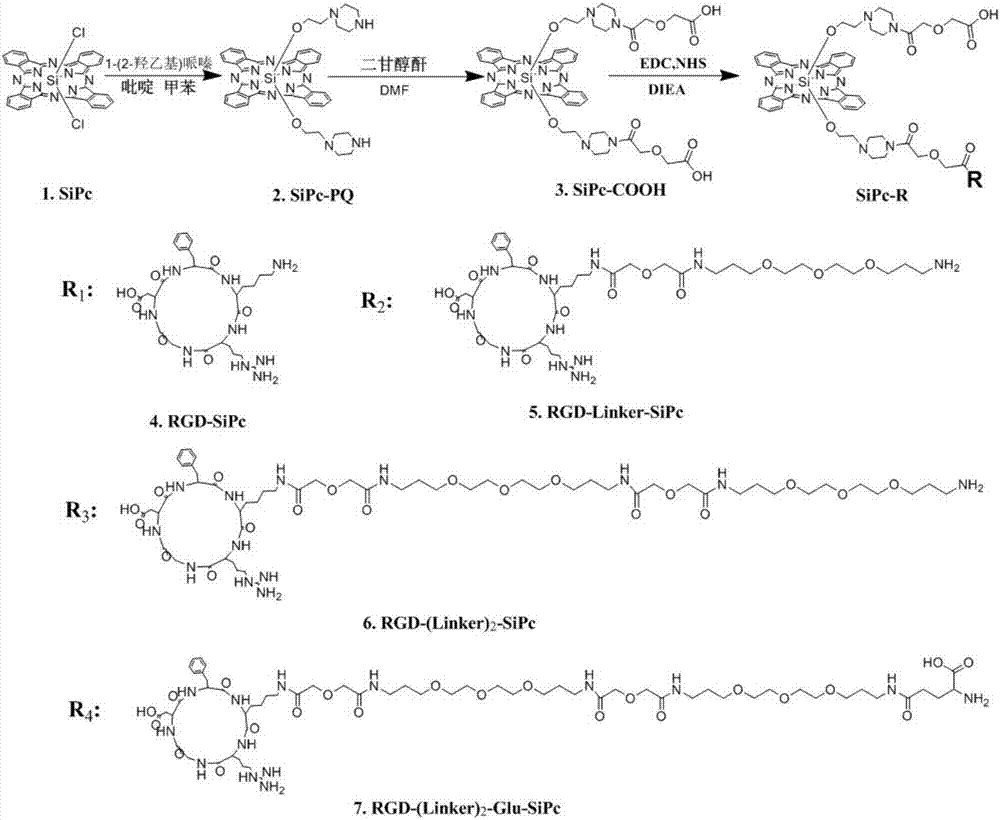
[0040] Embodiment 3 is abbreviated as RGD-SiPc, RGD-Linker-SiPc, RGD-(Linker) 2 -SiPc and RGD-(Linker) 2 -Synthesis of Glu-SiPc (attached image 3 )
[0041] 1. Synthesis of a compound called SiPc-PQ for short
[0042] 1-(2-Hydroxyethyl)piperazine (20.8mg, 0.016mmol) and SiPcCl 2 (10.0mg, 0.016mmol) was added to a mixed solution of toluene and pyridine (1mL, v / v=5:1) and refluxed for 10h, the solvent was removed under reduced pressure, and dichloromethane (CH 2 Cl 2 ) diluted, washed three times with saturated brine, combined the organic phases, added anhydrous sodium sulfate (Na 2 SO 4 ) was dried, the solvent was removed, and the silica gel column was purified, and the eluent was methanol (MeOH), dichloromethane (CH 2 Cl 2 ), and a small amount of triethylamine (TEA) was added to obtain the product SiPc-PQ (5.0 mg, yield 42%). (HRMS(ESI):m / z calculated for C 44 h 42 N 12 o 2 Si[M+H] + 799.3396, found 799.3392)
[0043] 2. Synthesis of the compound referred to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com