Cyclobenzaprine hydrochloride sustained-release tablet
A technology of cyclobenzaprine hydrochloride and sustained-release tablets, applied in the field of medicine, can solve the problems of prolonging biological half-life, reducing the number of times of taking medicines, slowing down the absorption rate, etc., and achieves the effects of low cost, maintaining blood drug concentration, and few times of taking medicines
Active Publication Date: 2016-03-16
CP PHARMA QINGDAO CO LTD
View PDF5 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0010] In order to solve the shortcomings of the existing cyclobenzaprine hydrochloride preparations, which are inconvenient to take and have low bioavailability, the present invention invents cyclobenzaprine hydrochloride sustained-release tablets, which can reduce the number of times of taking the medicine, slow down the absorption rate, prolong the biological half-life, and increase the blood drug concentration. Control within the effective blood concentration range, thereby reducing side effects and improving patient compliance
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
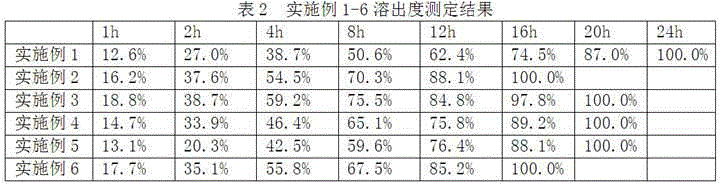
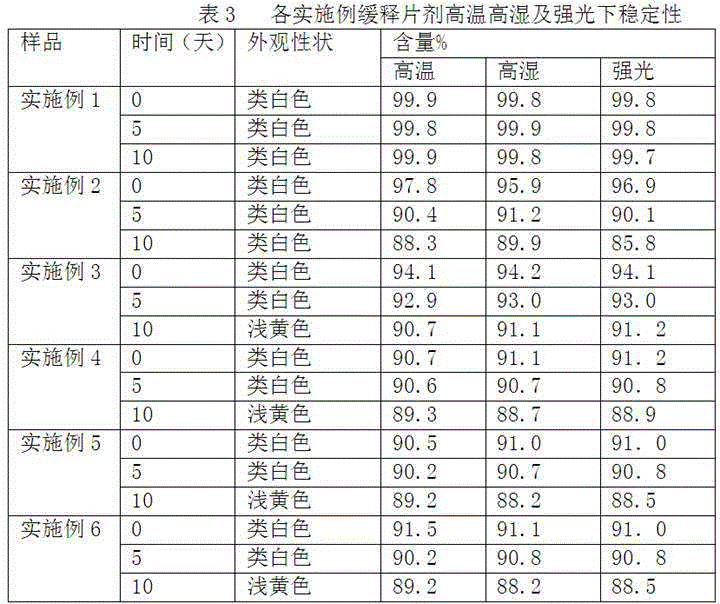
Embodiment 1-6
[0022] The preparation of embodiment 1-6 cyclobenzaprine hydrochloride sustained release tablet
[0023] According to the raw and auxiliary materials in the following table, the cyclobenzaprine hydrochloride sustained-release tablets of six examples were prepared according to the above-mentioned preparation method.
[0024]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention provides a preparation method of a cyclobenzaprine hydrochloride sustained-release tablet, and belongs to the technical field of a medicine. The cyclobenzaprine hydrochloride sustained-release tablet disclosed by the invention consists of cyclobenzaprine hydrochloride, a sustained-release material, a pore-foaming agent, a filling agent, a lubricating agent and distilled water. The cyclobenzaprine hydrochloride, as a major ingredient, has effects of relieving muscle spasm as well as accompanied severe pain in skeletal muscle and the like; and the provided sustained-release preparation is safe and effective, stable in quality, low in cost and low in administration efficiency, and the sustained-release preparation is capable of enhancing patient compliance and is capable of stably stopping pain and relieving spasm.
Description
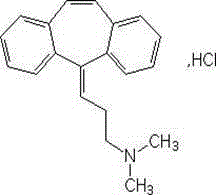
technical field [0001] The invention belongs to the technical field of medicine, and relates to a preparation method of cyclobenzaprine hydrochloride sustained-release tablets. The invention provides a safe and effective drug with stable quality, low cost, less frequency of administration, enhanced patient compliance, and stable pain relief. Antispasmodic sustained-release formulation. Background technique [0002] Cyclobenzaprine hydrochloride, the chemical name is 5-(3-dimethylaminopropene) dibenzo[a,e] cycloheptatriene hydrochloride, the structural formula is: [0003] [0004] Molecular formula: C 20 h 21 N·HCl [0005] Molecular weight: 311.85 [0006] Musculoskeletal system diseases are more common in clinical practice, involving bones, intervertebral discs, nerves, muscles, joints, soft tissues and other diseases. There are also many causes of musculoskeletal pain, such as inflammation, mechanical injury, immune reasons, tumors, etc. Musculoskeletal pain occur...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/22A61K31/135A61K47/38A61K47/36A61P21/02A61P29/00
Inventor 王明刚陈阳生任莉孙桂玉刘晓霞臧云龙翟翠云
Owner CP PHARMA QINGDAO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com