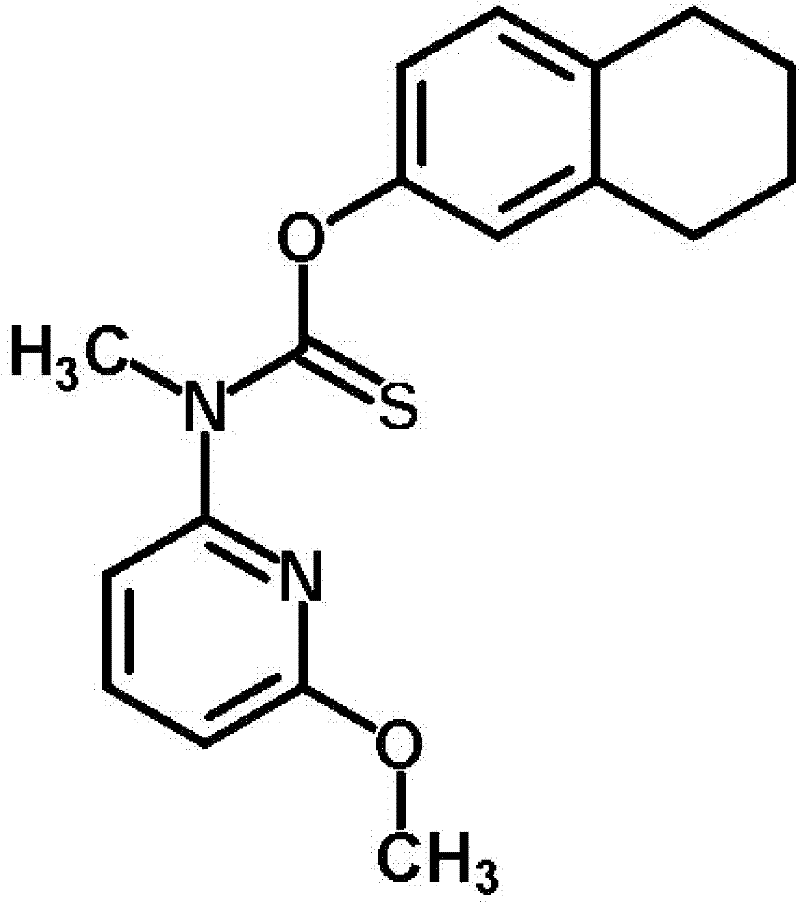
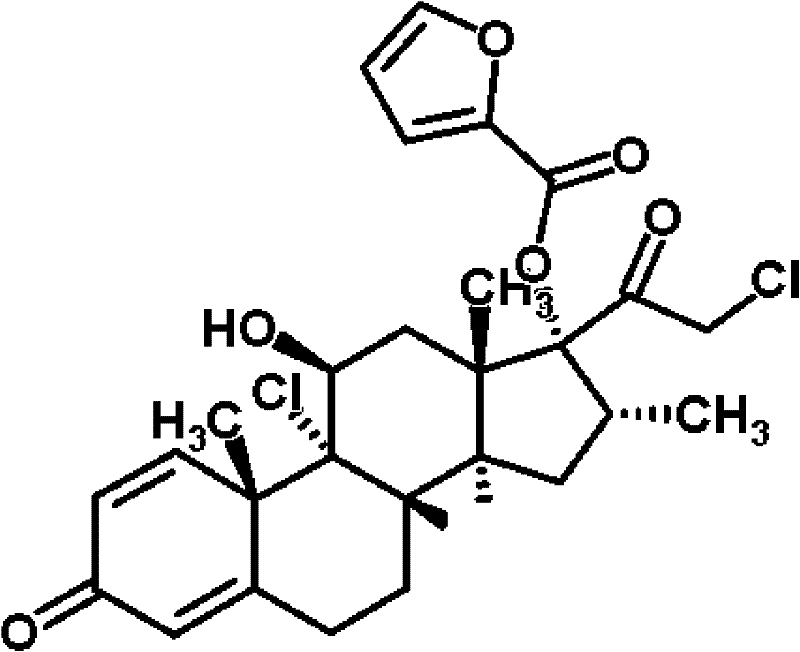
Liranaftate and mometasone furoate containing locally applied compound pharmaceutical composition
A technology for topical application of mometasone furoate, applied in the direction of drug combinations, medical preparations containing active ingredients, local antibacterial agents, etc., can solve the problems of obvious, easy to relapse, and ineffective curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, compound recipe liranaftate mometasone furoate cream
[0044] formula:
[0045] Main ingredients: liranaftate 0.5g, mometasone furoate 20g
[0046] Oil phase: 10g beeswax, 70g light liquid paraffin
[0047] Emulsifier: Cetearyl Alcohol 90g, Glyceryl Monostearate 15g, Sodium Lauryl Sulfate 10g
[0048] Preservatives: Ethylparaben 1g,
[0049] Water phase: propylene glycol 50g, distilled water 733.5ml
[0050] Preparation method: take cetearyl / stearyl alcohol, glyceryl monostearate, beeswax, light liquid paraffin, ethylparaben, and heat to dissolve into an oil phase. Separately take propylene glycol and distilled water and heat to 90°C, then add sodium lauryl sulfate to dissolve into water phase. Then slowly pour the water phase into the oil phase, and stir while adding until it cools down to obtain an emulsion matrix. Add liranaftate and mometasone furoate into the above matrix, and stir while adding until uniform, that is be made of.
Embodiment 2
[0051] Embodiment 2, compound recipe liranaftate mometasone furoate gel
[0052] formula:
[0053] Main ingredients: liranaftate 0.5g, mometasone furoate 20g
[0054] Gel base: Carbomer 12g
[0055] Complexing agent: Edetate disodium 0.5g
[0056] Preservative: Ethylparaben 2g
[0057] pH adjuster: Triethanolamine 7g
[0058] Water phase: propylene glycol 150g, ethanol 100g, distilled water 708ml
[0059] Preparation method: Sprinkle a small amount of carbomer into the aqueous solution of disodium ethylenediamine tetraacetate and propylene glycol under stirring. After it is completely dissolved, add triethanolamine solution and stir, then add liranaphthyl ester and molybdenum furoate Ethanol solution of metasone and ethylparaben. Mix well and serve.
Embodiment 3
[0060] Embodiment 3, compound recipe liranaftate mometasone furoate suspension
[0061] formula:
[0062] Main ingredients: liranaftate 20g, mometasone furoate 0.5g
[0063] Wetting agent: glycerin 200g
[0064] Suspending agent: sodium carboxymethylcellulose 5g
[0065] Solvent: water 775ml;
[0066] Preparation method: Liranaftate and mometasone furoate are ground into a paste with glycerin, and sodium carboxymethylcellulose is made into a paste, mixed with glycerin paste under stirring, added water to the full amount, and stirred evenly, that is have to.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com