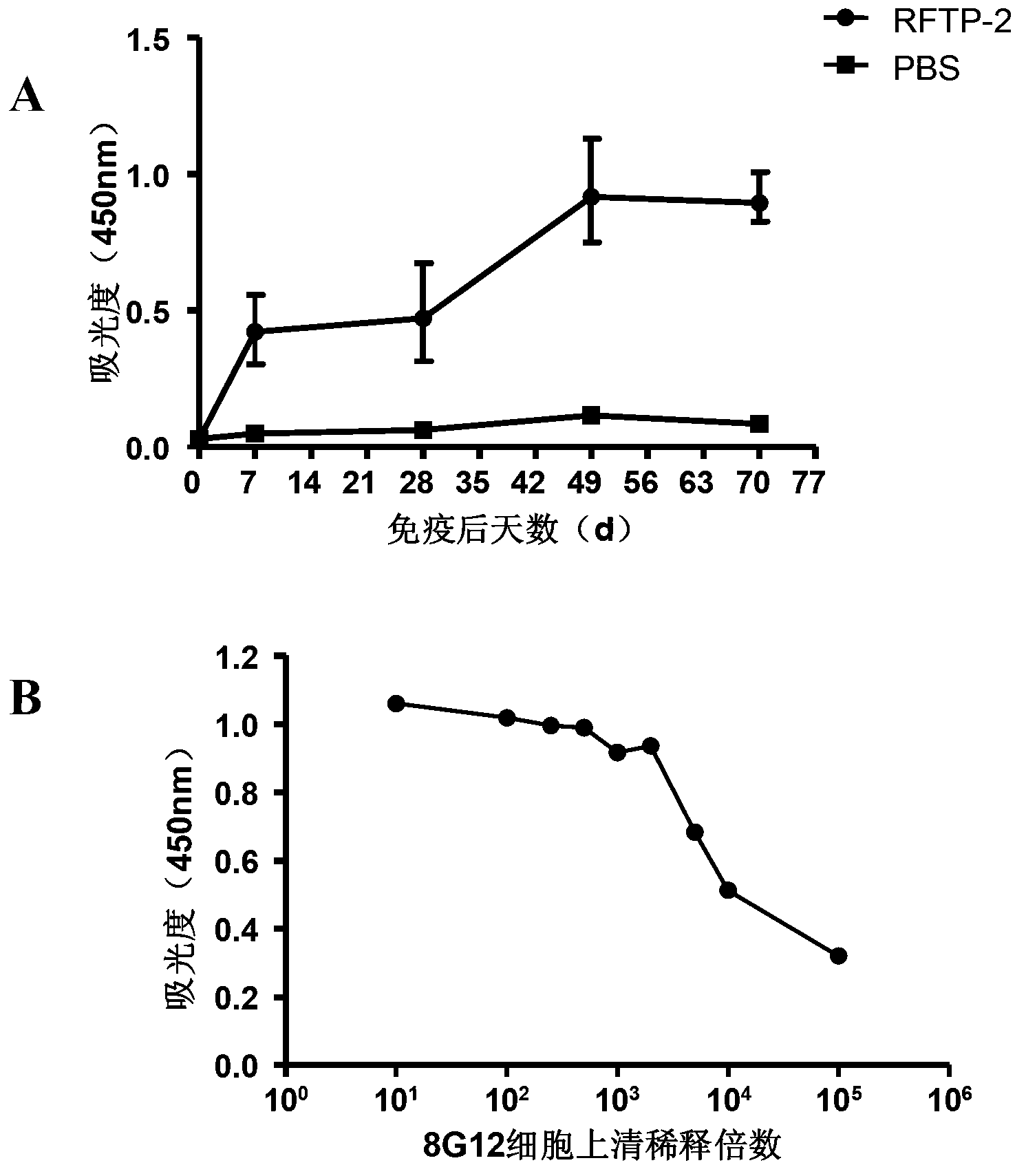
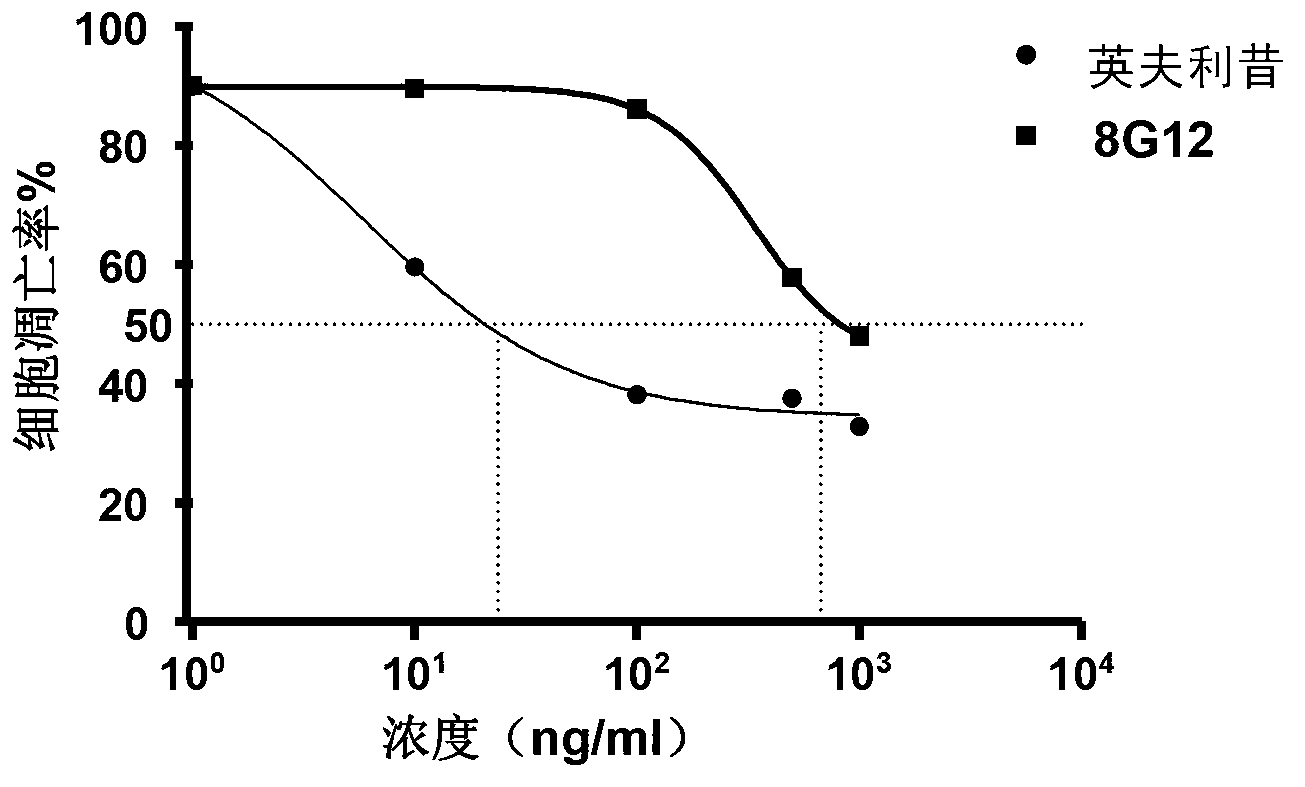
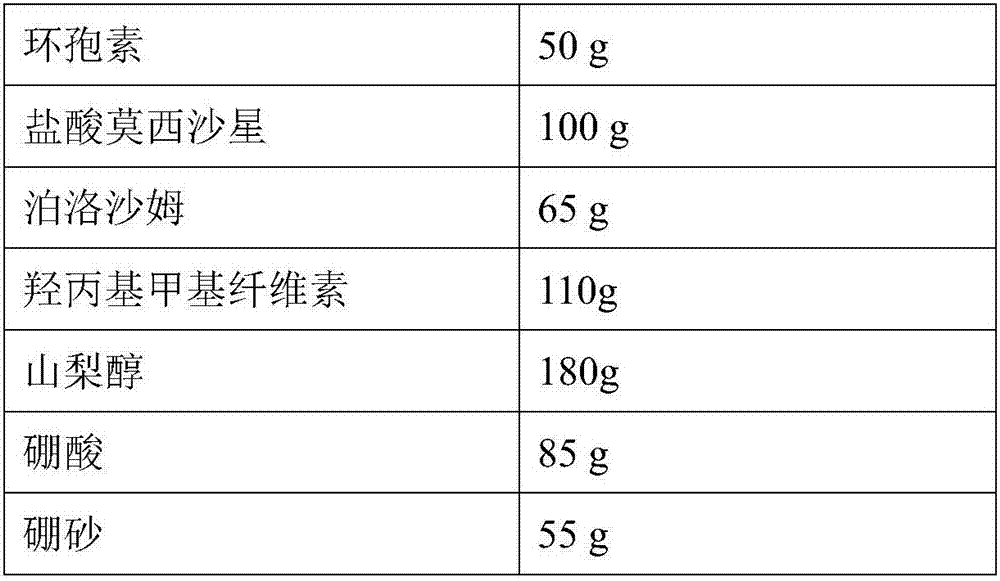
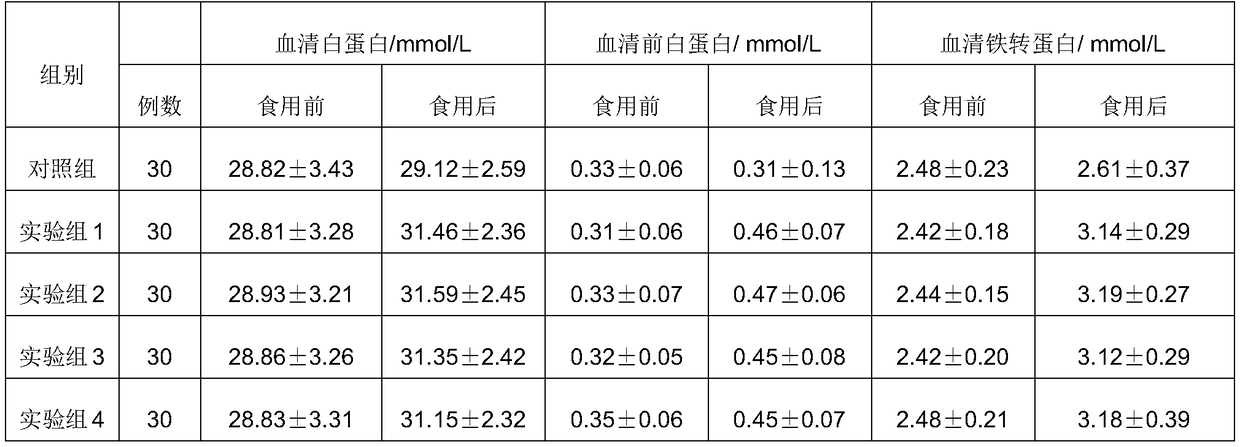
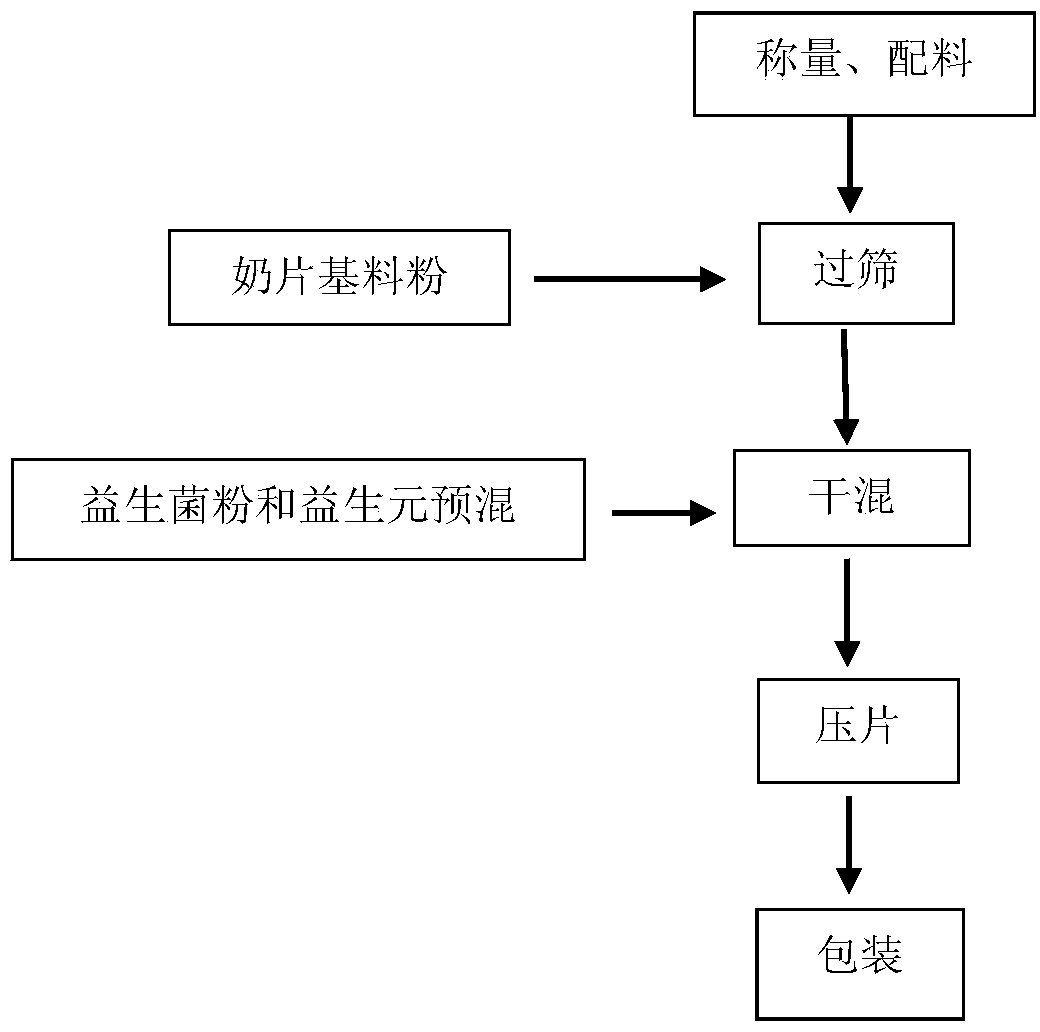
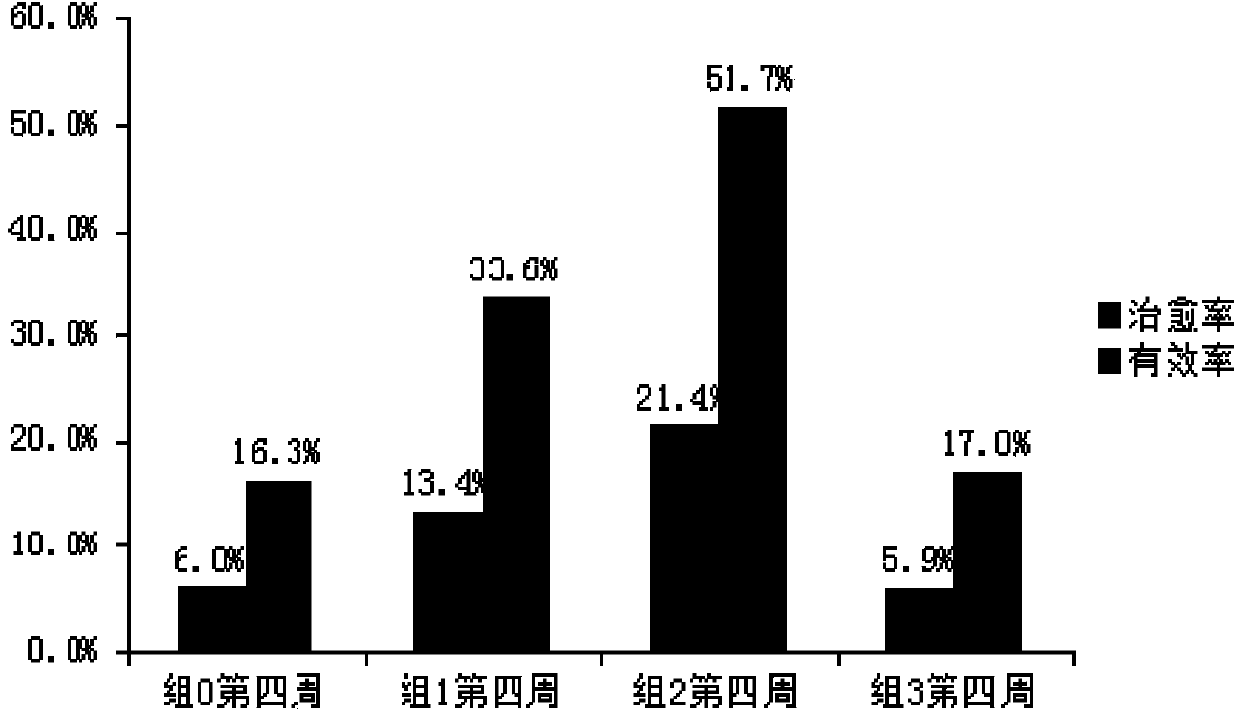
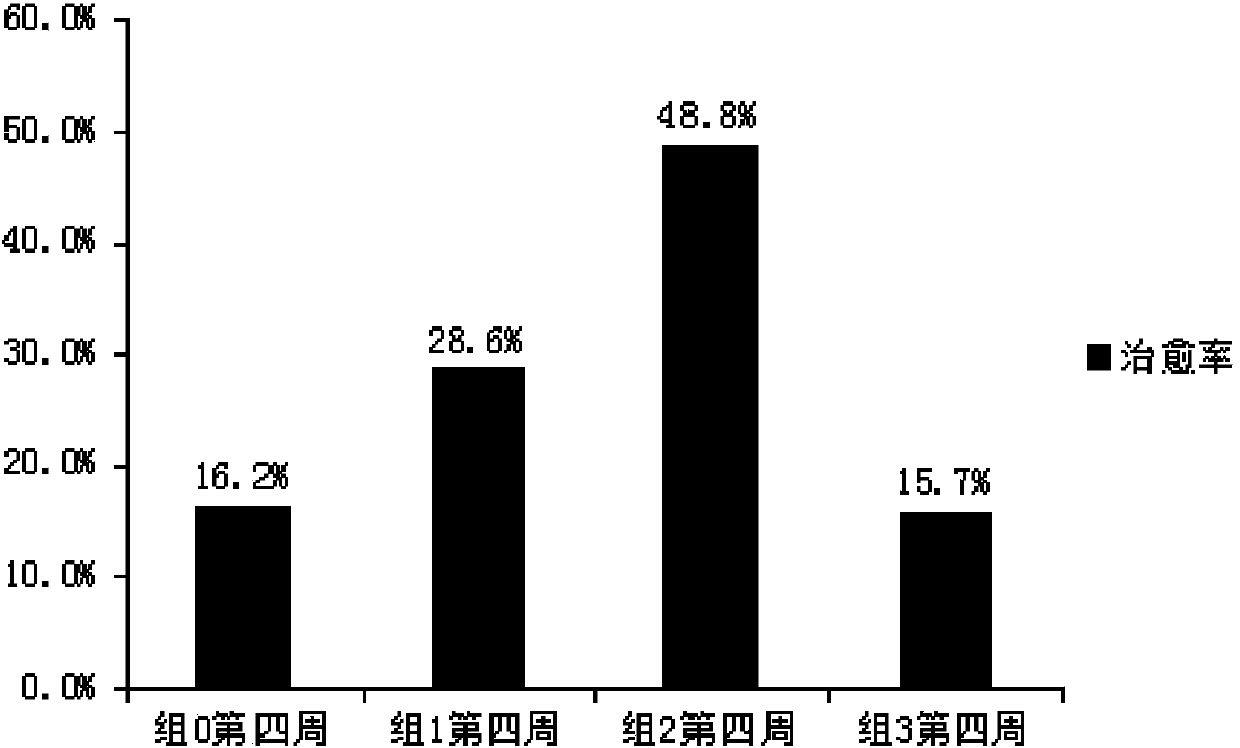
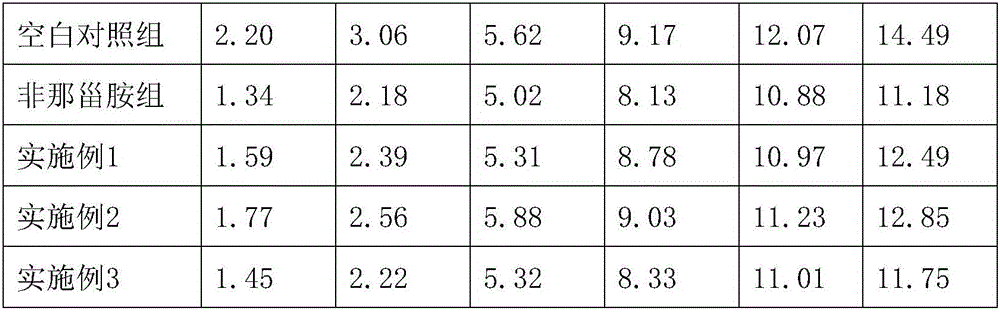
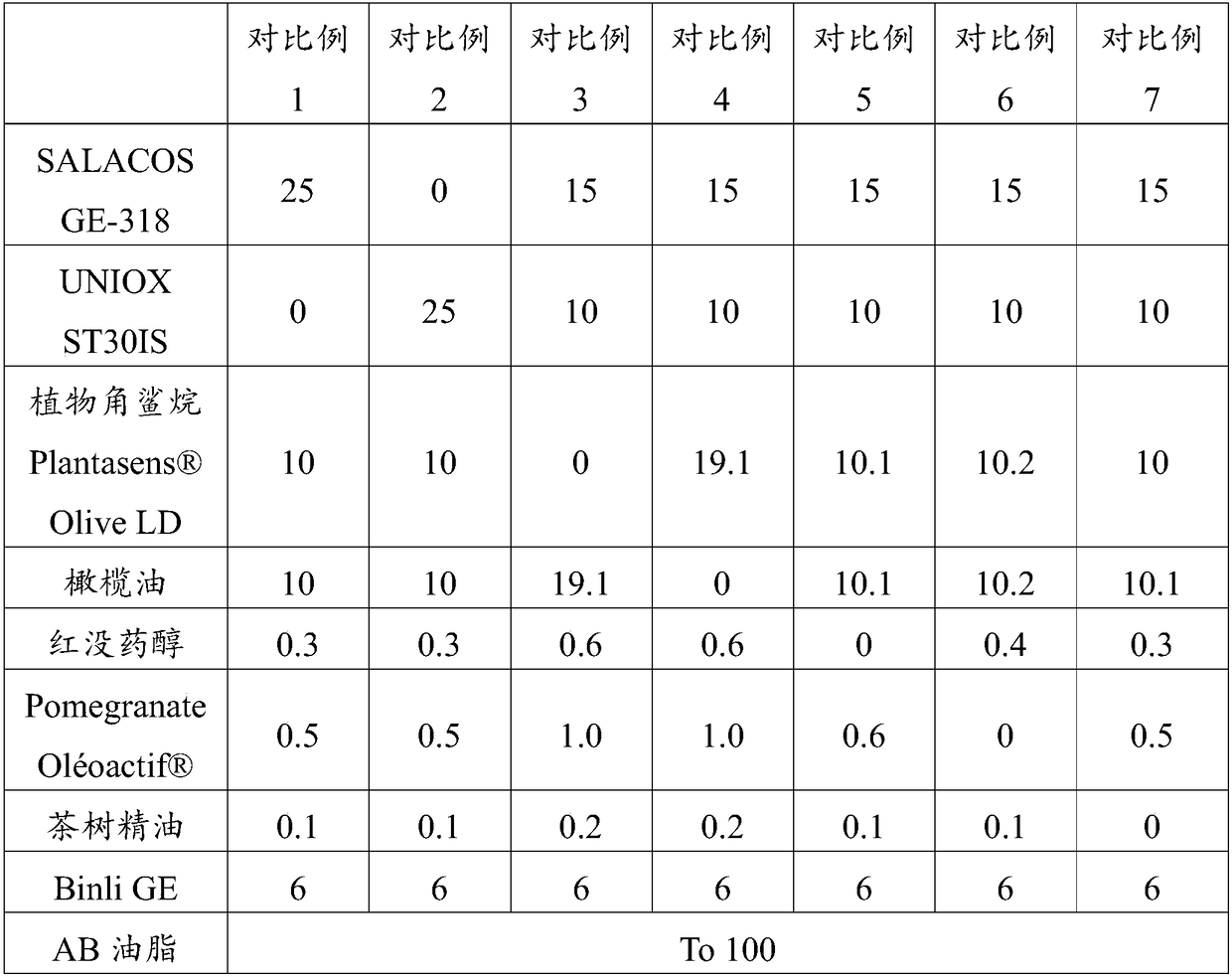
Patents
Literature
304results about How to "Relieve inflammation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
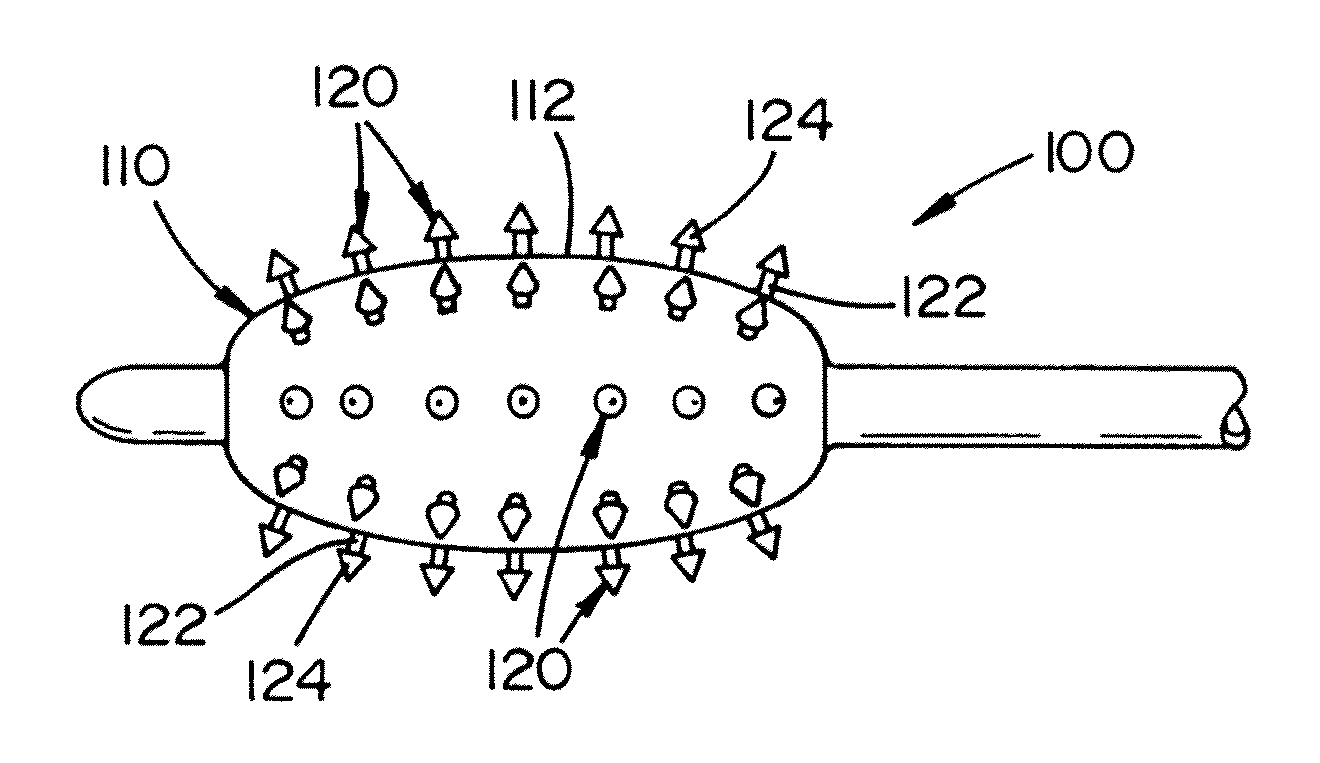
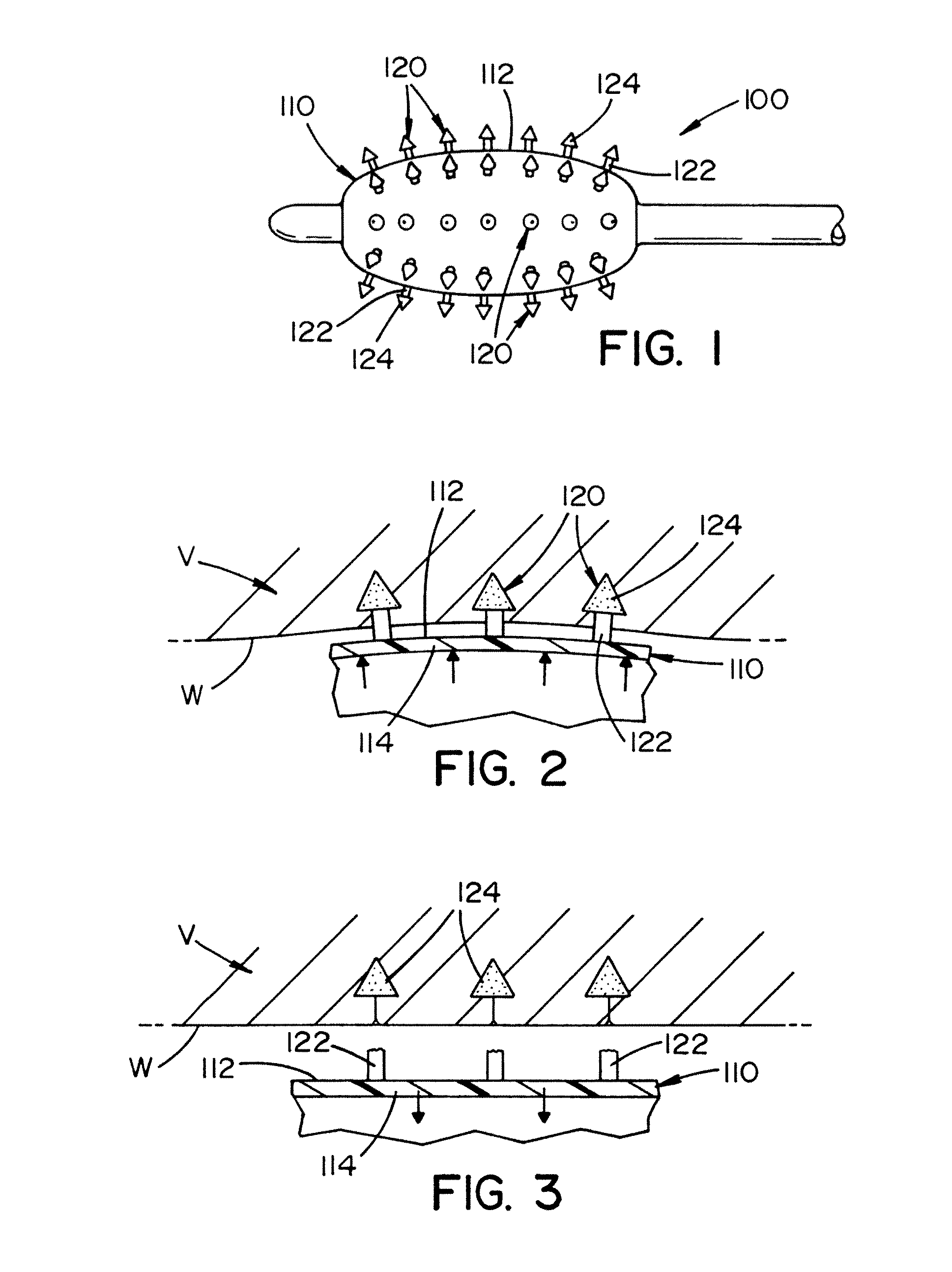
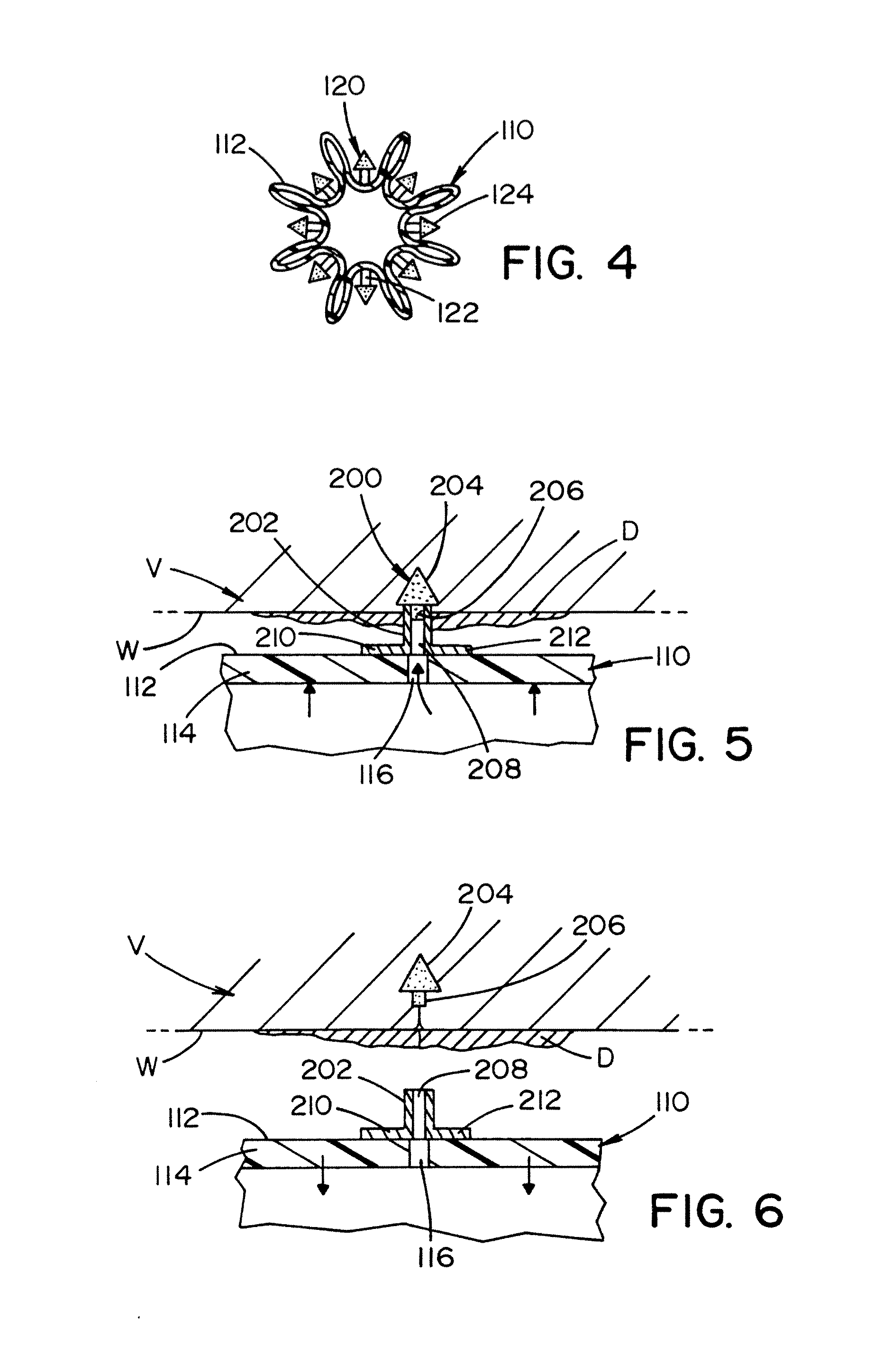
Biodegradable protrusions on inflatable device
ActiveUS20120041412A1Protection from damageFacilitated releaseStentsBalloon catheterSurgeryMedical device
A medical device for insertion and expansion in a body passageway. The medical device includes an inflatable device such as a balloon that is designed to be inflated and deflated while positioned in the body passageway. The inflatable device is inflatable by inserting a fluid in an internal cavity of the inflatable device. The inflatable device includes an outer surface that has a surface structure or micro-surface structure which is designed to at least partially penetrate into an inner wall of the body passageway when the inflatable device is inflated.
Owner:MIRUS LLC
Topical applications for skin treatment
InactiveUS20020039591A1Relieve inflammationPromote healingBiocideCosmetic preparationsMineral oilDisease
The present invention comprises novel combinations of mineral oil, vegetable shortening, vitamin E, purified water and other ingredients to form useful therapeutic, dermatological, pharmaceutical, medical or cosmetic compositions for treatment of skin maladies and disorders.
Owner:DAHLE JOSEPH SCOTT
Bifidobacterium longum and applications thereof
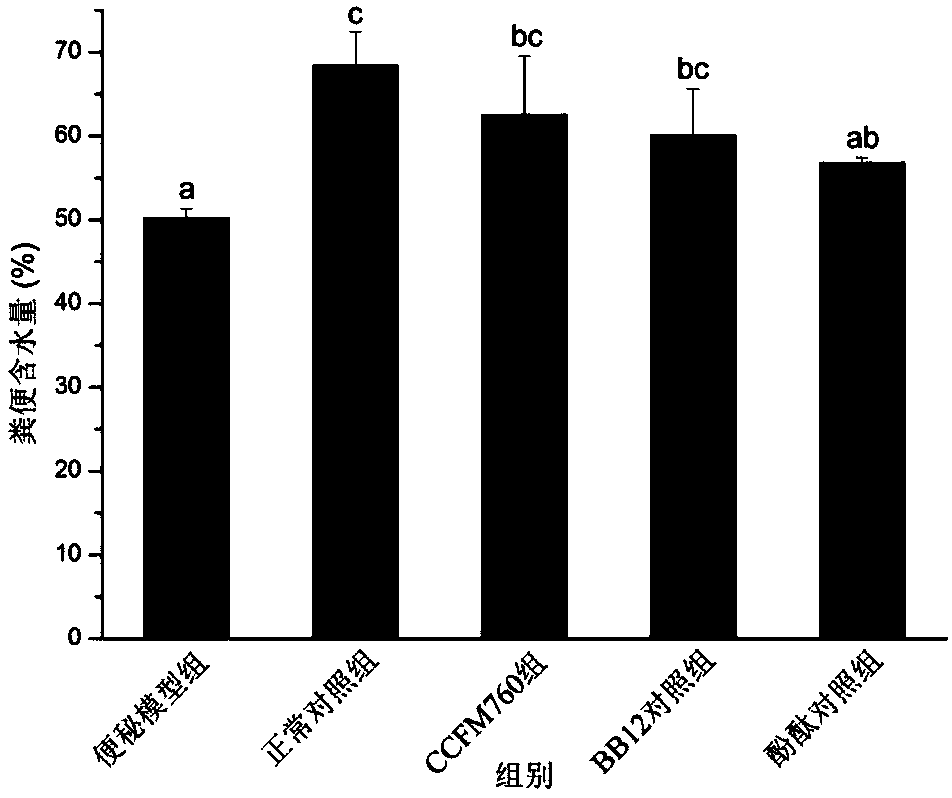
ActiveCN107893044AImprove toleranceGood growth and acid productionBacteriaDigestive systemPhenolphthaleinFeces
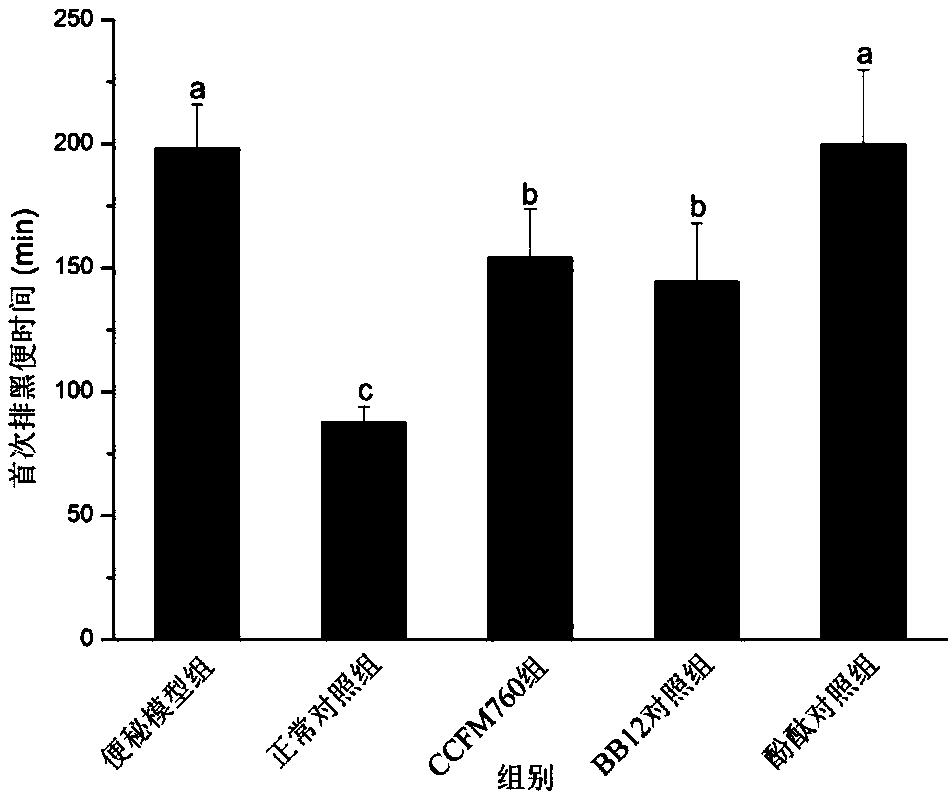
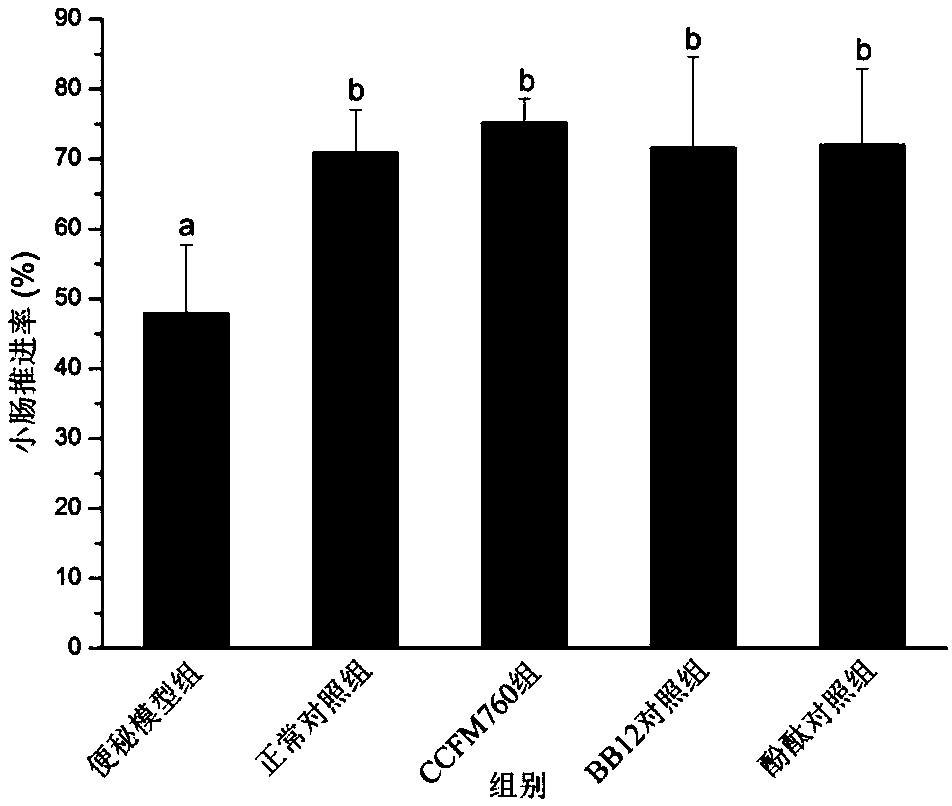
The invention discloses bifidobacterium longum and applications thereof, and belongs to the technical field of microorganisms. The bifidobacterium longum is deposited in the China General Microbiological Culture Collection Center in Institute of Microbiology, Chinese Academy of Sciences at number 3, No.1 yard, Beichenxi road, Chaoyang district, Beijing on December 7, 2017, and the accession numberis CGMCC NO.15032. The bifidobacterium longum has good growth, acid production, and acid resistant properties, and high adhesive capacity, can significantly increase the water content of faeces and the pushing rate of small intestine of mice with constipation, shortens the first black stool evacuation time, relieves inflammation of colonic mucosas, and regulates contents of gastrointestinal peptides related to constipation in serum. A constipation relieving effect of the bifidobacterium longum is equivalent to that of bifidobacterium animalis BB12, but an effect of the bifidobacterium longumon the pushing rate of small intestine is superior to those of bifidobacterium animalis BB12 and phenolphthalein. Accordingly, the bifidobacterium longum strain CCFM760 can be widely applied in various foods or medicine matrixes.
Owner:上海华朴生命健康科技有限公司
Medicament for treating acquired immune deficiency syndrome (AIDS)
InactiveCN102210837ARelieve spasmsReduce the burden onAmphibian material medical ingredientsAnthropod material medical ingredientsPollenForsythia
The invention provides a completely oral medicament for treating acquired immune deficiency syndrome (AIDS), comprising the following components: hedyotis diffusa, honeysuckle, meadowrueleaf corydalis root, dandelion, weeping forsythia, asparagus, ophiopogon root, figwort root, fresh rehmannia root, peach kernel, chuanxiong rhizome, zedoary, achyranthes root, dahurian patrinia herb, giant knotweed, stiff silkworm, isatis root, white peony root, polygonatum, bitter caramon, mistletoe, tortoise plastron, glossy privet fruit, oyster, pollen, salvia miltiorrhiza, tree peony bark, bupleurum, bitter orange, cimicifuga foetida, dried orange peel, amomum fruit, officinal magnolia bark, rose, songaria cynomorium herb, self-heal, medicine terminalia fruit, gromwell, licorice, chrysanthemum, castor bean, bullfrog gallbladder, baikal skullcap root, medicated leaven, malt, rice sprout, chicken's gizzard-skin, radish seed, scorch-fried crataegus, Chinese yam, astragalus root, bighead atractylodes rhizome, sealwort, dangshen, prepared rehmannia root, cornus fruit, tuckahoe, jujube, Chinese wolfberry, cistanche, dragon bone, oyster, epimedium, curculigo root, deerhorn glue, ginseng, donkey-hide glue, white fungus, angelica, spatholobus stem and turtle shell. The medicament provided by the invention not only can cure AIDS and has good therapeutic effect on AIDS, but also can prevent infection of AIDS.
Owner:肖嘉惠
Antifungal effects of Morinda citrifolia
InactiveUS20030225005A1Relieve inflammationCalming feelings of anxietyAntibacterial agentsBiocideBacteroidesAcetic acid
The present invention relates to antifungal and antibacterial activity of processed Morinda citrifolia products, as well as from various fractions of extracts from these processed products and the Morinda citrifolia L. plant, and related methods to determine mean inhibitory concentrations. In particular, the present invention relates to ethanol, methanol and ethyl acetate extracts from Morinda citrifolia L. and their inhibitory activities on common fungi and bacteria and the identification of mean inhibitory concentrations.
Owner:TAHITIAN NONI INT INC
Polypeptide composition capable of effectively improving acne and repairing skin damage
PendingCN109125706AInhibition of growth and reproductionAvoid drug resistanceCosmetic preparationsToilet preparationsCuticleBacillus acnes
The invention discloses a polypeptide composition capable of effectively improving acne and repairing skin damage. The polypeptide composition comprises antibacterial polypeptides, polypeptides capable of inhibiting inflammatory response and / or polypeptides capable of promoting collagen and keratin regeneration and repairing acne marks. The polypeptide composition has the advantages that the growth and propagation of propionibacterium acnes and staphylococcus aureus can be inhibited, inflammatory response can be inhibited, edema and couperose skin can be prevented, corium layer collagen synthesis is increased, the regeneration and reinforcing of dermal-epidermis junction (DEJ) area structure tissue can be promoted, epidermis cell differentiation and maturation can be promoted, acne marks can be effectively repaired, and effective acne improving and skin damage repairing can be achieved.
Owner:SHENZHEN WINKEY MEDICAL RES DEV
Traditional chinese medicine composition to treat rheumatoid arthritis and Preparation method thereof
InactiveUS20090148536A1Relieve inflammationImprove capillary permeabilityBiocideAnthropod material medical ingredientsJoint arthralgiaBlood flow
The present invention discloses a traditional Chinese medicine composition to treat Rheumatoid Arthritis and its preparation method. The composition is mainly comprised of the following crude drugs: ant, Radix Salviae Miltiorrhizae, Radix Aconiti Preparata, Radix Ginseng, Caulis Spatholobi, and Ramulus Cinnamomi, etc. According to pharmaceutical methods, various clinical acceptable dosage forms can be prepared of the composition of the present invention, including but not limited to one of the following dosage forms: tablets, capsules, pills, granules, suspension, dripping pills, oral liquid preparation, etc. The drug of the present invention has the functions of invigorating the kidney and spleen, promoting blood flow and clearing out the vein, expelling wind-evil and removing wetness, eliminating cold to stop pain. It can be effectively used in the treatment of lingering arthralgia with weak, arthralgia, intumesce and morning stiffness, numbness and stickiness, difficult to flex and extend, rigor and deforming, the rheumatism and rheumatoid arthritis with the above symptoms.
Owner:GUO LAIWANG +1
Composition for removing acnes and application of composition in cosmetics
InactiveCN105769707ARelieve inflammationEase skin repairCosmetic preparationsToilet preparationsPropanoic acidFeverfew extract
The invention discloses a composition for removing acnes and application of the composition in cosmetics. The composition is prepared from the following components: a paeony extract, a cucumber extract, a feverfew extract, a yellowish sophora root extract, a hamamelis virginiana extract, a lonicera tragophylla extract, tea tree leaf oil and oligosaccharide. The composition disclosed by the invention further comprises one or the combination of several of nicotinamide, panthenol, allantoin and oat kernel oil. The composition is suitable for acne type skins and helps users resist and relieve inflammation caused by exuberant sebum secretion, sebaceous gland obstruction and mass propagation of propionic acid acne bacillus and to repair the skin. According to the application of the composition for removing the acnes in medicines or cosmetics, the composition can be made into liquid, gel, emulsion or paste and other forms.
Owner:EURO STANDARD GUANGZHOU COSMETICS CO LTD
RANKL-TNF sample region mouse monoclonal antibody and its preparation method and use
The invention relates to a RANKL-TNF sample region mouse monoclonal antibody and its preparation method and use. The RANKL-TNF sample region mouse monoclonal antibody is extracted from a RANKL-TNF sample region mouse monoclonal antibody hybridoma cell strain and has the preservation number of CGMCC NO.6853. The RANKL-TNF sample region mouse monoclonal antibody and its related reagents can effectively neutralize TNF-alpha in vitro thereby inhibiting cell apoptosis caused by TNF-alpha, and neutralize RANKL in vitro thereby inhibiting osteoclast generation. The RANKL-TNF sample region mouse monoclonal antibody can effectively relieve an acute inflammation degree, improve bone mass of a mouse suffering from osteoporosis, improve a cancellous bone trabecula microstructure, and effectively improve osteoporosis symptoms.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Cyclosporine compound eye drops and preparation method thereof
ActiveCN107998399AImprove bioavailabilityHigh drug loadingOrganic active ingredientsSenses disorderTolerabilityTear secretion
The invention relates to a cyclosporine-containing composition and a preparation method thereof. The compound eye drops are prepared from cyclosporine, anti-inflammatory or antibiotic drugs, a surfactant, a stabilizer, a thickener, an isoosmotic adjusting agent, a pH adjusting agentr and water or oil, the preparation is a mixed suspension agent or an emulsion, and one or two of a high pressure homogenization method and a wet grinding technique is / are combined for use in the preparation method. The cyclosporine compound eye drops have the advantages that the ophthalmic tolerance is excellent, the drug utilization degree is high, the tear secretion of people suffering from xerophthalmia can be effectively improved, the tear film is stabilized, the anti-inflammation function of the anti-inflammatory or antibiotic drugs and the immunoregulation function of cyclosporine can be synergistically exerted, xerophthalmia can be effectively treated, and the compound eye drops have a better treatment effect compared with the separate application of cyclosporine.
Owner:NKD PHARMA CO LTD
Topical applications for skin treatment
InactiveUS6667045B2Promote healingRelief from itching and inflammationCosmetic preparationsBiocideDiseaseSkin treatments
The present invention discloses a topical composition consisting of combinations of mineral oil, vegetable shortening, vitamin E, purified water and other ingredients to form useful therapeutic, dermatological, pharmaceutical, medical or cosmetic compositions for treatment of skin maladies and disorders, namely eczema.
Owner:DAHLE JOSEPH SCOTT
Herbal mouthwash and preparation method thereof
InactiveCN103735473AHave the effect of preventing dental cariesEliminate bad breathCosmetic preparationsAnthropod material medical ingredientsDiseaseOral disease
The invention belongs to the technical field of daily health care, and particularly relates to herbal mouthwash and a preparation method thereof, the herbal mouthwash comprises the following components: by mass, 3-9 parts of green tea, 5-10 parts of stevia rebaudianum, 2-6 parts of borneol, 5-13 parts of honeysuckle, 10-15 parts of mulberry leaf, 4-9 parts of hemianthus micranthemoides, 10-25 parts of gallnut and 500-1000 parts of water. The herbal mouthwash can eliminate bad breath, clear the mouth filth, sterilize, remove dental plaque, prevent or inhibit various oral diseases, prevent tooth decay, improve periodontal diseases, and clean the oral cavity.
Owner:何小刚
Clinical nutritional formula special for kidney dialysis and preparation method thereof
InactiveCN108651988AImprove cleanlinessRepair functionVitamin food ingredientsLipidic food ingredientsOxygenKidney
The invention provides a clinical nutritional formula special for kidney dialysis. The clinical nutritional formula special for kidney dialysis comprises the following components in parts by weight: 15-25 parts of protein, 8-12 parts of fat, 50-54 parts of carbohydrate, 3-5 parts of dietary fiber, 0.3-0.6 part of macro-elements, 0.01-0.05 part of trace elements, 0.005-0.02 part of fat-soluble vitamin, 0.01-0.2 part of water-soluble vitamin, 0.05-1 part of dietary essence, 0.05-2 parts of medicinal and edible components, 0.1-3 parts of natural plant compounds, and 0.005-2 parts of new resourcefood; and the invention also provides a preparation method of the clinical nutritional formula. The clinical nutritional formula special for kidney dialysis provided by the invention can provide balanced nutrient substances for patients with renal dialysis, and can maintain the stability of the body quality of the patient, enhance the ability of immune response and the repair function, reduce inflammation, and improve the activity of scavenging oxygen free radicals and the endothelial cell function.
Owner:上海奥医生物医药科技有限公司
Probiotics food composition with function of relieving inflammation of throat and food
ActiveCN103798392AGood sore throat symptomsRelieves throat inflammationMilk preparationFood preparationLactobacillus salivariusThroat
The invention provides a probiotics food composition with a function of relieving inflammation of the throat and a food. The composition comprises Lactobacillus salivarius subsp.salicinius and Bifidobacterium animalissubsp.lactis, wherein the lactobacillus salivarius is preferably the lactobacillus salivarius with the preservation number of CCTCC No.M2011127, and the bifidobacterium animalis is preferably the bifidobacterium animalis with the preservation number of CCTCC No. 5470. The composition further comprises xylooligosaccharide and / or xylitol. The probiotics food composition provided by the invention can be added into various healthy foods and health-care foods such as acidified milk and milk powder candies, and can be used for effectively relieving inflammation of the throat, particularly chronic pharyngitis.
Owner:INNER MONGOLIA YILI INDUSTRIAL GROUP CO LTD
Anti-releasing compound flexible liposome with positive charges and preparation method thereof
InactiveCN106075390AHigh encapsulation efficiencyGuaranteed concentrationOrganic active ingredientsTripeptide ingredientsHair follicleHair loss
The invention discloses an anti-releasing compound flexible liposome with positive charges and a preparation method thereof. The anti-releasing compound flexible liposome is prepared from the following components in parts by weight: 0.1-10 parts of linoleic acid, 5-30 parts of berberine hydrochloride, 5-30 parts of glycyrrhizic acid, 2-15 parts of glutathione, 0.01-5 parts of phytosphingosine, 1-40 parts of matrine and 1-25 parts of tea polyphenols. By researching a multi-target formula which has a clear mechanism and aims at androgenetic alopecia, an anti-hair loss composition is obtained; and the composition is prepared into the compound liposome with the positive charges, so that the flexible application of the composition on a preparation is improved, and adsorption of the preparation on scalps and deep osmosis of the preparation into hair follicles are promoted. The multi-target compounding effect of the preparation can effectively inhibit further development of hair loss, and the growth of new hairs is promoted.
Owner:GUANGDONG PHARMA UNIV
Mild dandruff-removing and itching-relieving composition as well as preparation method and application thereof
ActiveCN108969424AImprove anti-dandruff effectRelieve inflammationCosmetic preparationsHair cosmeticsAlcoholPiroctone olamine
The invention discloses a mild dandruff-removing and itching-relieving composition. The mild dandruff-removing and itching-relieving composition is prepared from the following components in percentageby weight: 2 to 10 percent of piroctone olamine, 2 to 15 percent of an auxiliary dandruff-removing composition component, 5 to 40 percent of an alcohol type dissolving-assisting component, 1 to 15 percent of an anti-inflammatory and itching-relieving component and the balance of water. According to the mild dandruff-removing and itching-relieving composition disclosed by the invention, the piroctone olamine is compounded with the auxiliary dandruff-removing composition component with the specific type and the specific compounding ratio to use, and is compound with the anti-inflammatory and itching-relieving component with the specific type to use; the prepared composition can be used for effectively improving the dandruff-removing effect and also can be used for alleviating inflammation problems of scalps; the composition is added into a shampoo formula, so that the dandruff-removing effect can be realized and the scalp nursing effect also can be realized.
Owner:HUAANTANG BIOTECH GRP CO LTD
Soothing anti-allergic cosmetic composition
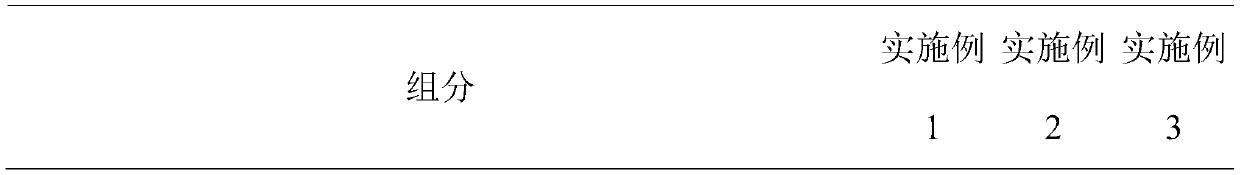
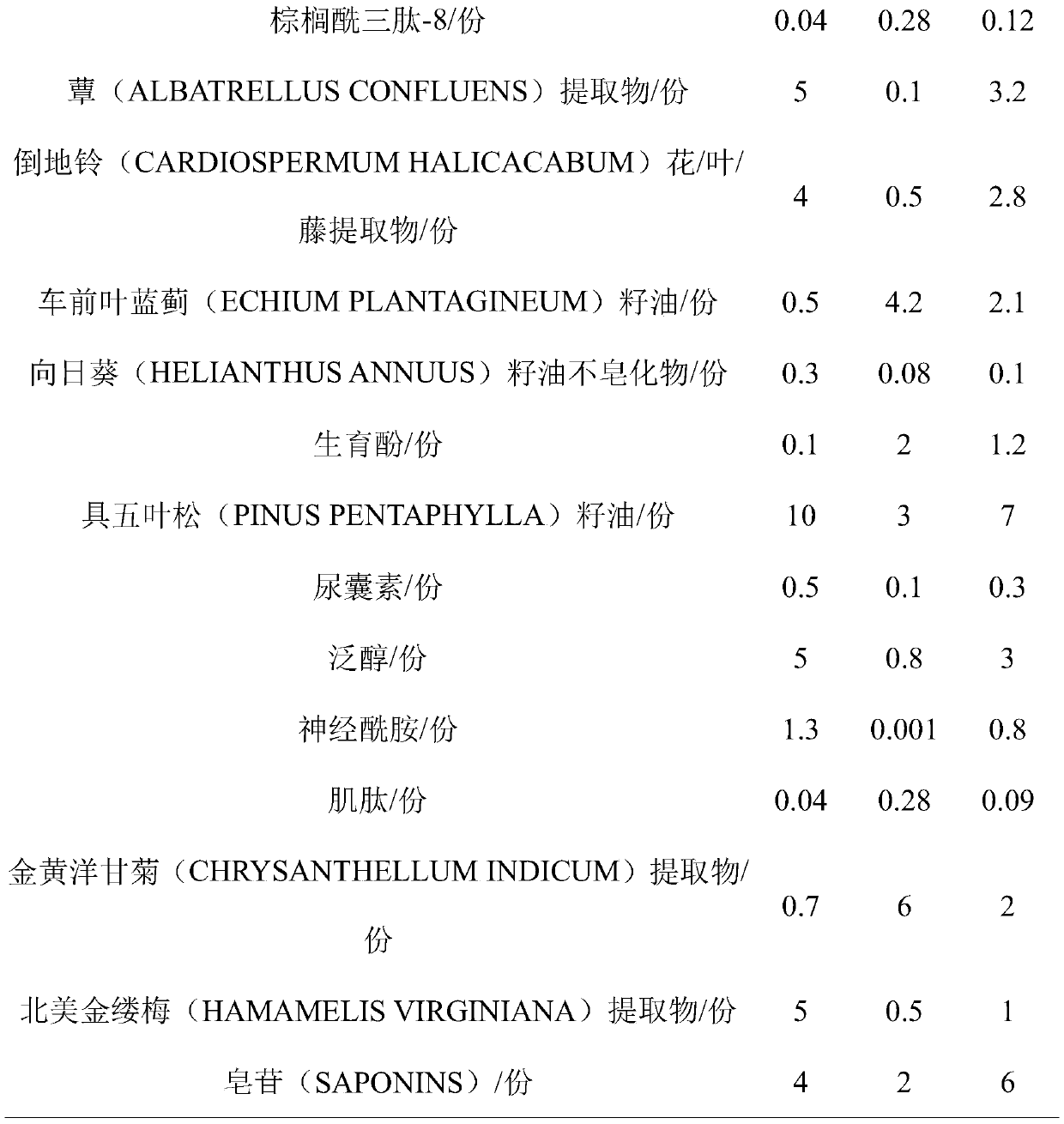
ActiveCN111494266APrevent and relieve inflammation and symptomsSoothe the skinCosmetic preparationsToilet preparationsSunflower seedChrysanthellum indicum
The invention belongs to the technical field of cosmetics, and relates to a soothing anti-allergy cosmetic composition. The composition comprises the following components by weight: 0.04-0.28 part ofpalmitoyl tripeptide-8; 0.1-5 parts of a mushroom extract; 0.5-4 parts of a cardiospermum halicacabum flower / leaf / vine extract; 0.5-4.2 parts of echium plantagineum seed oil; 0.08-0.3 part of sunflower seed oil unsaponifiable matter; 0.1-2 parts of tocopherol; 3-10 parts of pinus pentaphylla seed oil; 0.1-0.5 part of allantoin; 0.8-5 parts of panthenol; 0.001-1.3 parts of ceramide; 0.04-0.28 partof carnosine; 0.7-6 parts of a chrysanthellum indicum extract, 0.5-5 parts of a hamamelis virginiana extract and 2-6 parts of saponin. The soothing anti-allergy cosmetic composition contains differenteffective components in reasonable proportions, all the active components cooperate with one another, sensitive skin can be improved and repaired, stimulation of parts of exogenous factors to the skin can be effectively blocked, and burning heat, stabbing pain, pruritus and taut feeling and other symptoms are not further induced any more.
Owner:广州雅纯化妆品制造有限公司
Sun cream containing liposome component of hydrolyzed pearl solution
ActiveCN101278894AAvoid damageRelieve inflammationCosmetic preparationsToilet preparationsNutrientChemistry
The invention discloses a sun cream comprising the liposome component of the hydrolyzed pearl solution, and is prepared by 2-ethylhexyl-4-methoxy cinnamate, caprylic / capric triglyceride, simethicone, tert-butyl-methoxyl dibenzoyl methane, 3, 4'-methyl benzal camphor, titanium dioxide, phenoxyethanol, compound emulsifying wax, polyglycerol methyl acrylate, polyethylene glycol (7) glycerol ether, polyacrylic acid, the liposome of the hydrolyzed pearl solution, perfume composition, imidazolidinyl urea and deionized water. The sun cream has the advantage of simple technique, and effectively combines a plurality of amino acids and microelements in the liposome of the hydrolyzed pearl solution with other components, which not only effectively prevents UVA / UVB from damaging the skin, relieves a skin inflammation caused by sunburn, but also promotes the skin regeneration, heals the damaged skin, ensures the skin to be moistened, and provides a nutrient necessary for the skin, thereby realizing the double effect of sun block and whitening.
Owner:HAINAN JINGRUN PEARL BIOTECH
Dendranthema morifolium nanotechnology skin care product and preparation method and application thereof
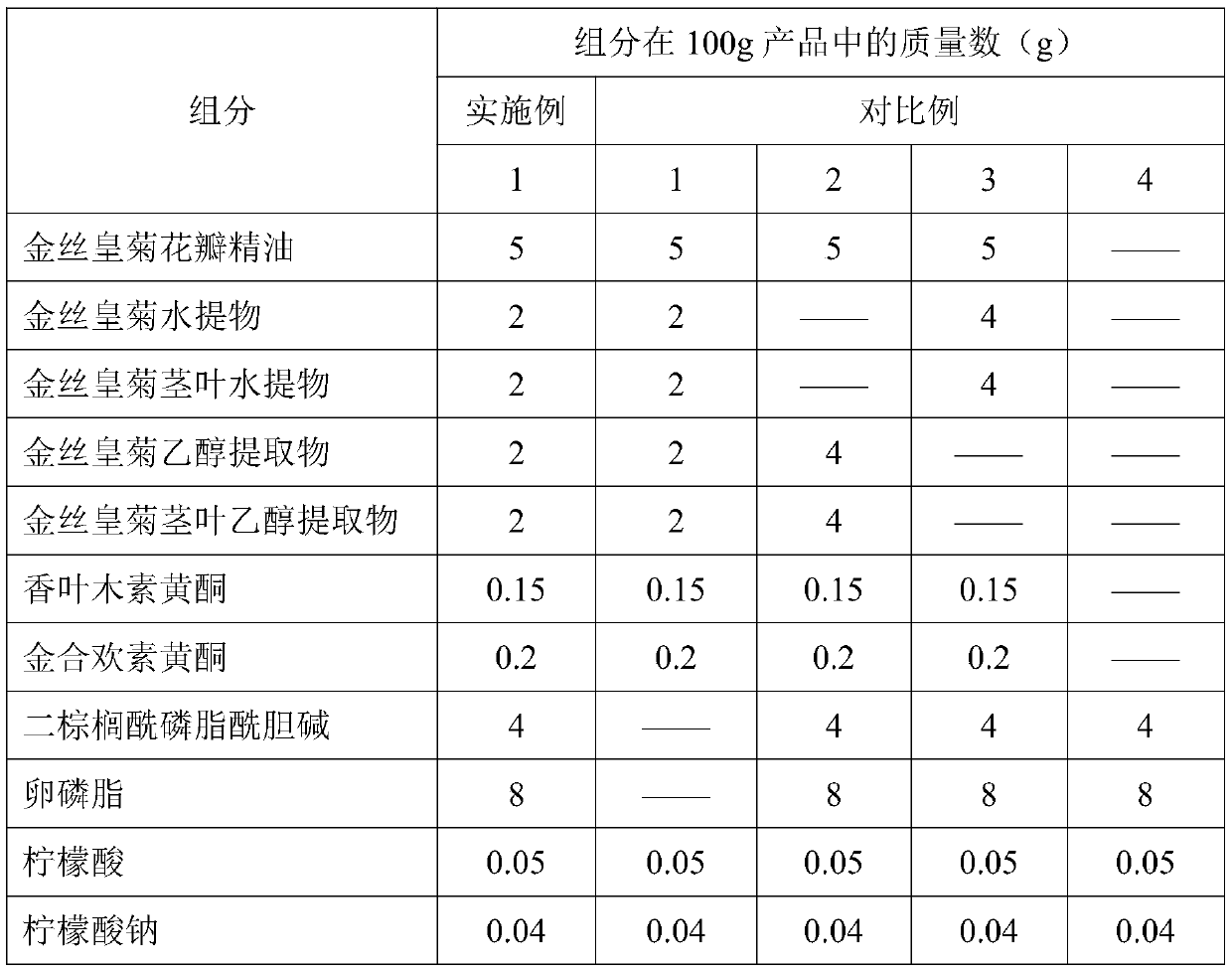
ActiveCN111481481AUltimate skin care effectOptimal Screening of Nanotechnology Process ParametersCosmetic preparationsAntipyreticAcacetinPolyethylene glycol
The invention relates to a Dendranthema morifolium nanotechnology skin care product which is composed of the following raw materials: Dendranthema morifolium petal essential oil, a Dendranthema morifolium aqueous extract, a Dendranthema morifolium alcohol extract, a Dendranthema morifolium stem and leaf aqueous extract, a Dendranthema morifolium stem and leaf alcohol extract, and diosmetin and acacetin flavones separated from Dendranthema morifolium; dipalmitoyl phosphatidylcholine, lecithin, polyethylene glycol 400, hyaluronic acid, trehalose, glyceryl stearate, sodium methyl hydroxybenzoate,polysorbate-20, citric acid, sodium citrate, sodium carbonate and distilled water supplemented to 100 parts. According to the nanotechnology skin care product added with multiple active ingredients of Dendranthema morifolium, multiple components and proportions are reasonably and scientifically designed, nanotechnology process parameters are optimized and screened, and the skin care effect of Dendranthema morifolium is brought into play to the maximum extent.
Owner:江苏灵源沂岸科技股份有限公司
Nano-emulsion with good stability
ActiveCN109453043AImprove stabilityAuxiliary emulsification is goodCosmetic preparationsToilet preparationsTocopheryl acetateChemistry
The invention discloses a nano-emulsion with good stability. A formula of the nano-emulsion is prepared from the following components in percentage by weight: 30.0-82.13 percent of purified water, 0.01-0.1 percent of EDTA disodium, 0.5-5.0 percent of hydrogenated lecithin, 0.3-5.0 percent of a nonionic surfactant, 0.5-5.0 percent of caprylic acid / caprinic acid triglyceride, 5.0-30 percent of squalane, 1.0-5.0 percent of isononyl isononanoate, 0.01-0.5 percent of tocopheryl acetate, 0.5-3.0 percent of 1,2-hexanediol, 10.0-40.0 percent of glycerin and 0.05-8.0 percent of an oil-soluble skin conditioner. Through reasonable selection and ratio blending of components of the nano-emulsion, the strength and the elasticity of an interfacial film are improved, the interface energy is lowered, the mobility of the interfacial film is reduced, and the nano-emulsion with the stability to temperature change is prepared. The nano-emulsion wraps various efficient skin-care components and promotes transdermal absorption of functional components; and in addition, the hydrogenated lecithin as an emulsifier has better physiological activity, promotes substance metabolism of epidermic cells and enablesskin to be more pliable, tougher and more elastic; and modified soyasterol has the effect of relieving skin inflammation.
Owner:NOX BELLCOW COSMETICS CO LTD
Natural plant extract composition and preparation method and application thereof
ActiveCN105832611AMaintain activitySun protection skin inflammationCosmetic preparationsToilet preparationsWater soluble chitosanMoisture
The invention belongs to the technical field of cosmetics, and relates to a natural plant extract composition and a preparation method and application thereof. The natural plant extract composition is prepared from, by weight, 8-15 parts of plant extract microcapsules, 3-10 parts of Chinese holly leaf extracting solution and 5-12 parts of dandelion extracting solution. The plant extract microcapsules are prepared from, by weight, 3-8 parts of rhizoma curcumae longae extract, 1-3 parts of folium eriobotryae extract, 2-6 parts of emblic leafflower fruit extract, 0.5-3 parts of herba rabdosiae extract, 0.5-2 parts of polyglycerol ester, 15-30 parts of common bletilla tuber polysaccharide and 8-15 parts of water-soluble chitosan. According to the natural plant extract composition, the synergistic effect of the microcapsule technology and multiple kinds of plant extract is applied, therefore, the activity of all active ingredients can be effectively kept, and meanwhile the remarkable effects of whitening the skin and preserving moisture, blocking the sun, restoring the skin after basking and the like are achieved.
Owner:GUANGDONG COOWAY BIOTECH CO LTD
Use of modified polysaccharide rich in mannose in preparing medicine for preventing the colitis cancer
InactiveCN101306012APrevent cancerRelieve inflammationOrganic active ingredientsDigestive systemGut immunityUlcerative colitis
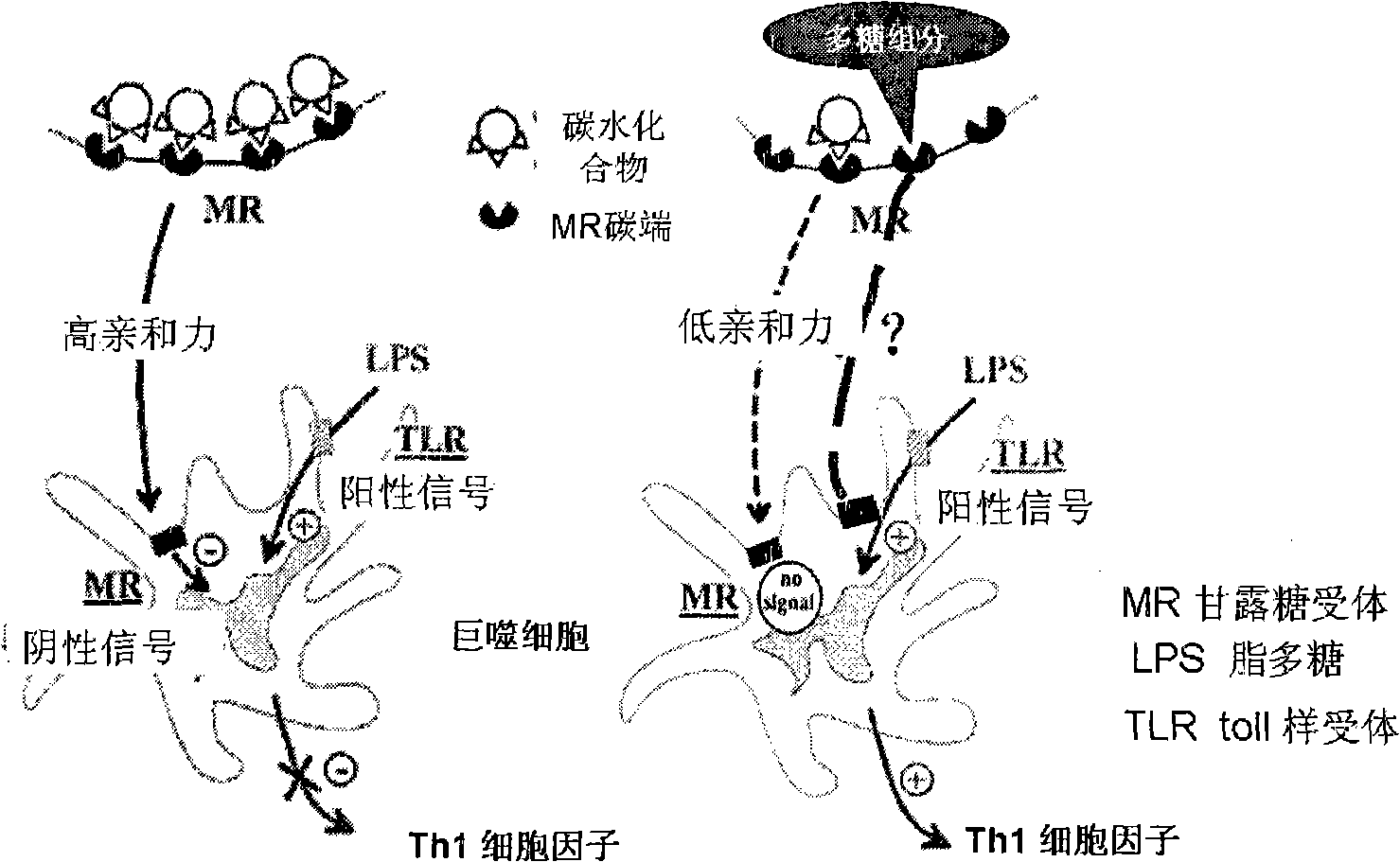
The invention relates to the application of a mannose-enriched polysaccharide to drug preparation, in particular to the application of a mannose-enriched modified polysaccharide to the preparation of drugs for preventing colonitis from cancerization. Aiming to make up the blank of the drugs for preventing the inflammatory bowel diseases and especially the ulcerative colitis from cancerization, the application provides the technical proposal that the mannose-enriched modified polysaccharide is applied to the drugs for preventing the colonitis from cancerization, and the modified polysaccharide contains a modified natural gum or a modified plant polysaccharide. The application has the characteristics that a mannose receptor (MR) is taken as a target; the mannose-enriched polysaccharide is separated and extracted from a medicinal plant, and mannosidase is adopted for hydrolyzation; mannose residues are exposed, and regulate gut immunity and relieve the intestinal inflammation after being combined with the MR on the surface of immunological cells, thereby preventing the inflammatory bowel diseases and especially the ulcerative colitis from cancerization. After the mannose-enriched modified polysaccharide is used on a big mouse suffering from the ulcerative colitis, the cancerization inhibitory rate of the ulcerative colitis can reach 93 percent.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Absorbable medical suture line with good mechanical performance
ActiveCN105079869AImprove mechanical propertiesHigh strengthSuture equipmentsLactidePolyethylene glycol
The invention discloses an absorbable medical suture line with good mechanical performance. The absorbable medical suture line comprises, by weight, 100-120 parts of modified polylactic acid, 20-40 parts of polyglycollide, 4-6 parts of sorbitol, 3-5 parts of methyl methacrylate and 5-6 parts of cassia oil. The preparation method of the modified polylactic acid includes: in ice-bath, adding chitosan into perchloric acid, well mixing, dripping sorbic acid chloride, stirring, cooling, preserving heat and purifying to obtain modified chitosan; taking polyethylene glycol, feeding inert gas, heating, preserving heat until the polyethylene glycol melt, adding stannous chloride and lactide, well mixing, heating, preserving heat and stirring, and purifying to obtain intermediate material; dissolving the modified chitosan in mixed solvent, adding triethylamine, well mixing, heating, refluxing, adding the intermediate material, well mixing, continuing refluxing, purifying and drying to obtain the modified polylactic acid.
Owner:安徽省康宁医疗用品有限公司
Healthcare agent for pet joints and preparation method thereof
InactiveCN106620657APromote recoveryRelieve inflammationPeptide/protein ingredientsSkeletal disorderArthritisFeed additive
The invention discloses a healthcare agent for pet joints and belongs to the technical field of feed additives. The healthcare agent uses chondroitin sulfate, glucosamine hydrochloride, dimethyl sulfone and collagen as the core components and vitamins and mineral elements as the auxiliary materials and is applicable to pets with arthritis, caused by excessively fast growth and development or maldevelopment, movement damage, senile joint degeneration and the like, of different degrees. The healthcare agent can promote bone health, increase pet immunity, improve pet bone joint diseases and increase pet living quality.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Oil-control acne-removing functional composition and gel
PendingCN112426386ANo side effectsGood effectAntibacterial agentsCosmetic preparationsCentella asiatica extractBacillus acnes
The invention relates to an oil-controlling and acne-removing functional composition and gel. The oil-controlling and acne-removing functional composition is prepared by compounding five raw materialsincluding mangosteen peel extract, officinal magnolia bark extract, pomegranate peel extract, amur corktree bark extract and centella asiatica extract according to a certain proportion. Therefore, the natural, mild and non-irritant efficient oil-control acne-removing composition is formed. The gel prepared from the composition has the advantages of being mild to skin, free of irritation, free ofside effects, environmentally friendly, outstanding in effect and the like, the acne problem caused by pore blockage and excessive grease secretion can be relieved, and meanwhile the activity of propionibacterium acnes and inflammation induced by propionibacterium acnes are effectively inhibited, secretion of grease is regulated and controlled, bacteria are inhibited, abnormal keratosis is resisted, and skin inflammation is relieved.
Owner:天津尚美化妆品有限公司
Makeup removing stick and preparation method thereof
InactiveCN109248105AStrong emulsifying abilityImprove cleaning powerCosmetic preparationsMake-upIrritationTocopherol
The invention discloses a makeup removing stick and a preparation method thereof. The makeup removing stick comprises the following components of a compound of PEG-20 glyceryl triisostearate and tocopherol and sorbitol-30 tetraisostearate, and the weight ratio of the compound of the PEG-20 glyceryl triisostearate and the tocopherol to the sorbitol-30 tetraisostearate is (2-4):(1-3). The makeup removing stick has excellent emulsifying ability and high cleaning strength, can effectively remove cosmetics on the skin, is gentle to the skin without irritation, does not damage the skin keratin, hasskin repairing and moisturizing effects, provides various nutrition ingredients for the skin, has good spreadability, is fresh and not sticky in skin feeling, can further effectively alleviate skin allergies and inflammation, tightens skin pores, and has dual effects of makeup removing and skin care. The makeup removing stick is in a solid stick shape, has thixotropic properties, can spread quickly when the makeup removing stick contacts the skin, can fully dissolves makeup, convenient to use, easy to carry, and good in makeup removing effect.
Owner:I&B GUANGZHOU BIOLOGICAL TECH CO LTD
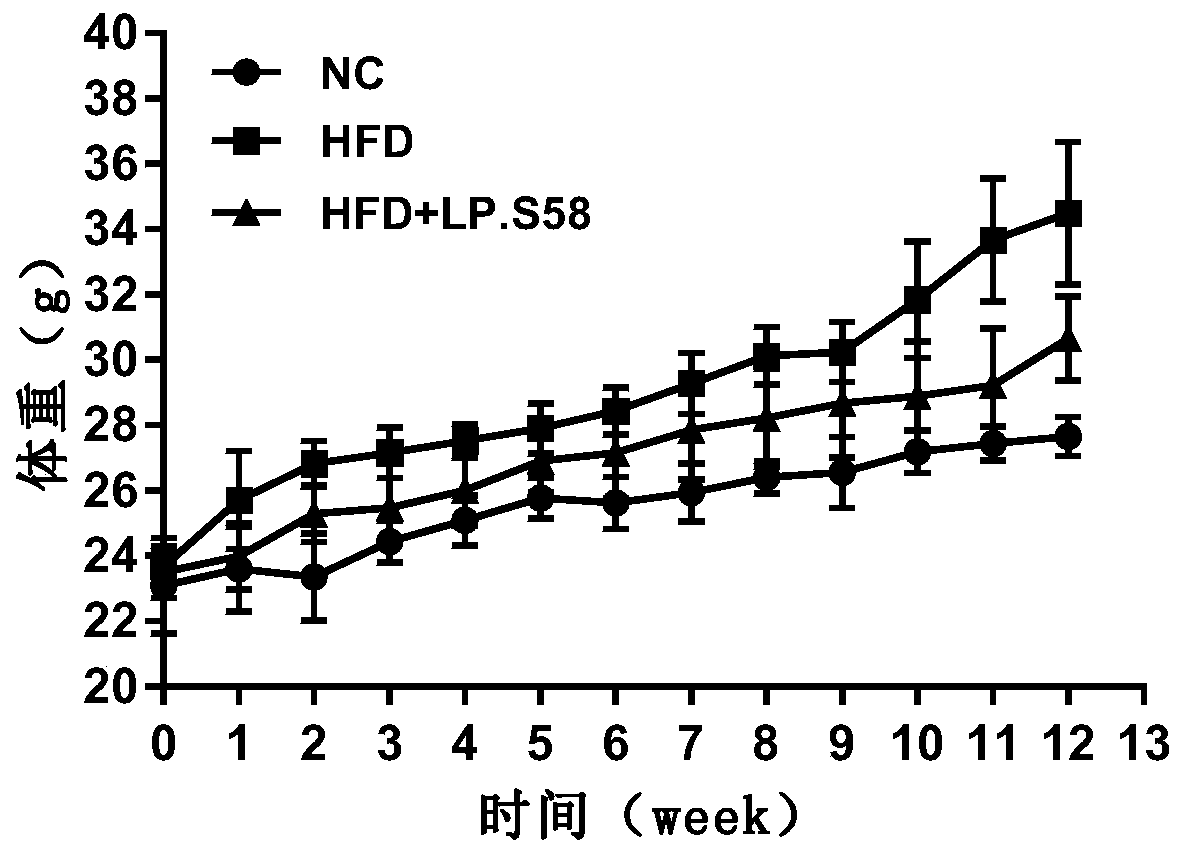
Lactobacillus plantarum S58 and application thereof in preparation of products for relieving obesity
The invention discloses a preservation number of CCTCC NO: M 2019595 Lactobacillus plantarumS58 and application thereof in preparation of products such as health care products, foods and medicines forrelieving obesity. The invention not only expands the application range of the Lactobacillus plantarumS58 and improves the utilization value of the Lactobacillus plantarumS58, but also brings new hope for treating obesity.
Owner:SOUTHWEST UNIVERSITY
Compound essential oil for preventing and treating psoriasis as well as preparation method thereof
InactiveCN103054989ASuitable for useEffective and safeCosmetic preparationsToilet preparationsArray data structureMedicine
The invention relates to a compound essential oil for preventing and treating psoriasis as well as a preparation method thereof and belongs to the technical field of cosmetics and medicines. The compound essential oil is composed of the following components in parts by volume: 500-900 parts of base oil, 3-9 parts of frankincense essential oil, 3-9 parts of pogostemon cablin essential oil, 3-9 parts of myrrh essential oil, 3-9 parts of chamomile blue essential oil, 3-9 parts of lavender essential oil, 1-5 parts of lemon essential oil and 0.5-4 parts of needle juniper essential oil. The compound essential oil has the advantages that multiple types of plant essential oils are scientifically combined to achieve the synergistic effect and well prevent and treat psoriasis, and the treatment cost is reduced.
Owner:YUNNAN YINAZI BIOTECH
Bubble hair conditioner
PendingCN113750020AGood antibacterial effectRelieve inflammationCosmetic preparationsHair cosmeticsActive agentEngineering
The invention belongs to the field of daily chemicals. A bubble hair conditioner comprises the following components in percentage by mass: 1-2% of saponin, 0.1-11% of a nonionic surfactant, 0.1-3% of a cationic surfactant, 0.1-18% of a humectant, 1-10% of proline, 0.1-1% of lactic acid, 0.1-1% of silicone oil, 0.1-5% of a plant extract and the balance of water. The hair conditioner disclosed by the invention has good film-forming property, foaming property and conditioning property, is rich and fine in foam, easy to clean, mild and low in irritation, reduces surface activity residual quantity, enables the skin to feel fresh and cool and not to be sticky and greasy, enables hair to be soft and fluffy and not to be greasy after being used, and can effectively repair hair cuticles, improve hair glossiness, smoothness and tensile strength, replenish water, preserve moisture, regulate scalp water-oil balance, and maintain the scalp environment steady state.
Owner:澳彩生物科技(惠州)有限公司
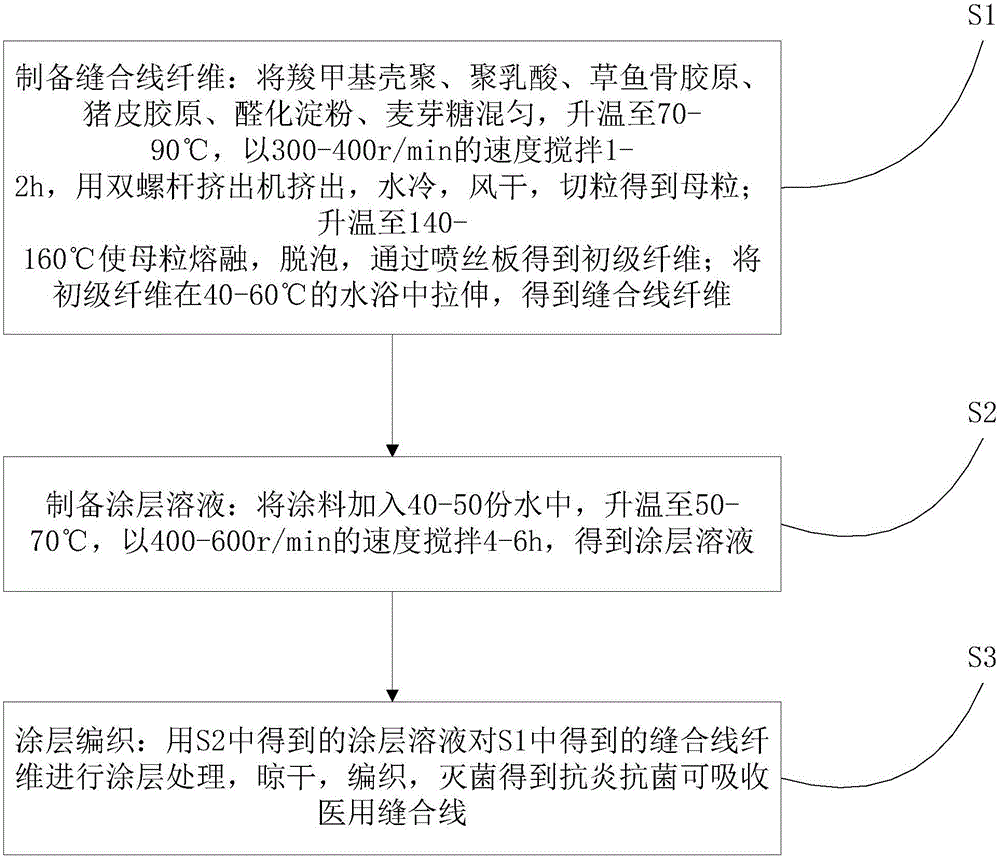
Anti-inflammatory, anti-bacterial and absorbable medical suture and preparation method thereof
ActiveCN105056295AImprove hydrophilicity and cell affinityImprove mechanical propertiesSuture equipmentsConjugated synthetic polymer artificial filamentsAnti-inflammatoryBiological tissue
The invention discloses an anti-inflammatory, anti-bacterial and absorbable medical suture comprising raw materials in parts by weight as follows: 80-100 parts of carboxymethyl chitosan, 10-20 parts of polyactic acid, 10-15 parts of grass carp bone collagen, 8-12 parts of pig skin collagen, 4-6 parts of aldehyde starch, 2-4 parts of maltose and 1-5 parts of a coating. The invention further discloses a preparation method of the anti-inflammatory, antibacterial and absorbable medical suture. The method comprises steps as follows: S1, preparing suture fibers; S2, preparing a coating solution; S3, performing coating treatment and knitting. The suture is good in anti-inflammatory and antibacterial performance, well compatible with biological tissue, good in mechanical performance and simple to operate, cells can adhere and reproduce on the suture easily, and industrial production is facilitated.
Owner:安徽省康宁医疗用品有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com