Cyclosporine compound eye drops and preparation method thereof
A technology of cyclosporine and eye drops, which is applied in the direction of cyclic peptide components, pharmaceutical formulas, emulsion delivery, etc., can solve the problems of poor interaction stability of nano-suspensions, and achieve good pharmacokinetic data and specific area Increases the effect of tear secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、025
[0049] The preparation of embodiment 1,0.25% cyclosporine compound eye drops (nanosuspension)
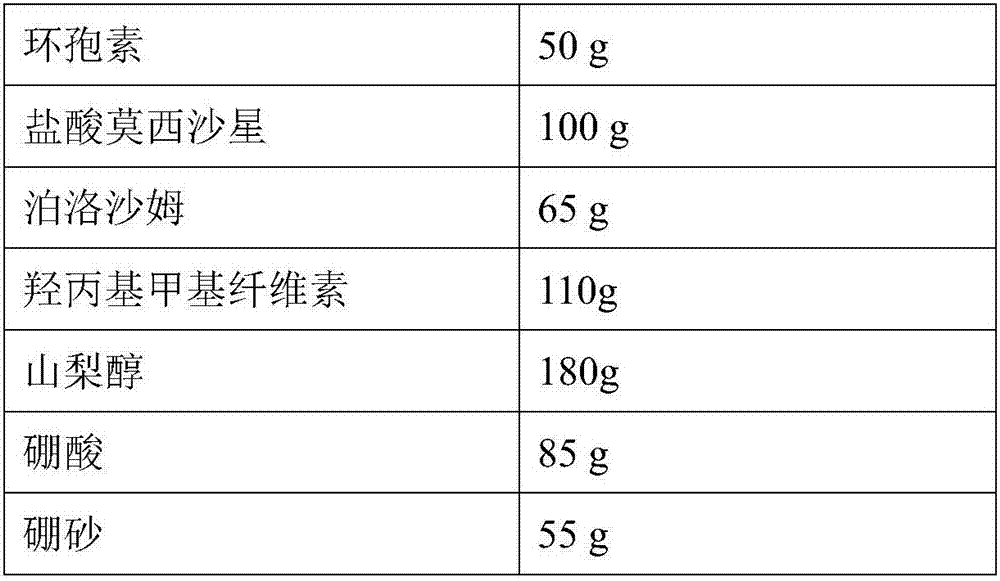
[0050] prescription
[0051]
[0052]
[0053] Preparation:
[0054] (1) Weigh 65g of poloxamer and dissolve it in 500mL of water for injection, add 50g of cyclosporine, stir and suspend evenly, then transfer to a wet grinding machine, using 0.6-0.8mm zirconia balls as the grinding medium, 1500rpm Grind for 20 minutes to obtain a nano-suspension, the average particle size of which is 850nm through particle size testing;
[0055] (2) Weigh 100g of moxifloxacin hydrochloride, 110g of hydroxypropyl methylcellulose, 180g of sorbitol, 85g of boric acid, 55g of borax, add 4L of water for injection, stir, dissolve and mix evenly, and filter through a 0.22μm filter membrane to obtain Inflammatory or antibiotic drug aqueous solution;
[0056] (3) Mix the cyclosporin nanosuspension prepared in step (1) with the anti-inflammatory or antibiotic drug aqueous solution obtained in step (2...
Embodiment 2、1
[0058] The preparation of embodiment 2, 1% cyclosporine compound eye drops (nanosuspension)
[0059] prescription
[0060] Cyclosporine
200g
Dexamethasone Sodium Phosphate
4.0g
Span 80
145g
400g
350g
glycerin
240g
Appropriate amount
Water for Injection
Add to 20L
[0061] Preparation:
[0062] (1) Weigh 145g of Span 80 and dissolve it in 1L of water for injection, add 200g of cyclosporine, stir and suspend evenly, transfer to a wet grinding machine, use 0.6-0.8mm zirconia balls as the grinding medium, and grind at 3000rpm After 60 minutes, the nanosuspension was obtained, and the average particle diameter was 450nm through the particle size test;
[0063] (2) Weigh 4.0g of dexamethasone sodium phosphate, 400g of polyvinylpyrrolidone K30, 350g of mannitol, 240g of glycerin, add 10L of water for injection, stir to dissolve and mix evenly, ...
Embodiment 3
[0066] The preparation of embodiment 3, 0.05% cyclosporine compound eye drops (nanosuspension)
[0067] prescription
[0068] Cyclosporine
10g
Indomethacin
100g
Tween 80
25g
polyethylene glycol 4000
90g
86g
12g
sodium hyaluronate
100g
Water for Injection
Add to 20L
[0069] Preparation:
[0070] (1) Weigh 25g of Tween 80 and dissolve it in 200mL of water for injection, add 10g of cyclosporine, stir and suspend evenly, and homogenize under 1000bar high pressure 12 times to obtain a nanosuspension, the average particle diameter of which is 235nm through particle size testing; The distribution is narrower, 150-300nm, in contrast, the API particles are larger, and the particle size distribution range is wider, 1-30μm.
[0071] (2) Weigh 100g of indomethacin, 90g of polyethylene glycol 4000, 86g of sodium chloride, 12g of citric acid, add 5L of water for injection, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com