Anti-inflammatory, anti-bacterial and absorbable medical suture and preparation method thereof
A suture and solution technology, applied in the field of medical suture, can solve the problems of skin tissue inflammation, wound bacterial infection, etc., to increase mechanical strength and cell affinity, increase antibacterial and anti-inflammatory properties, and increase mechanical properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
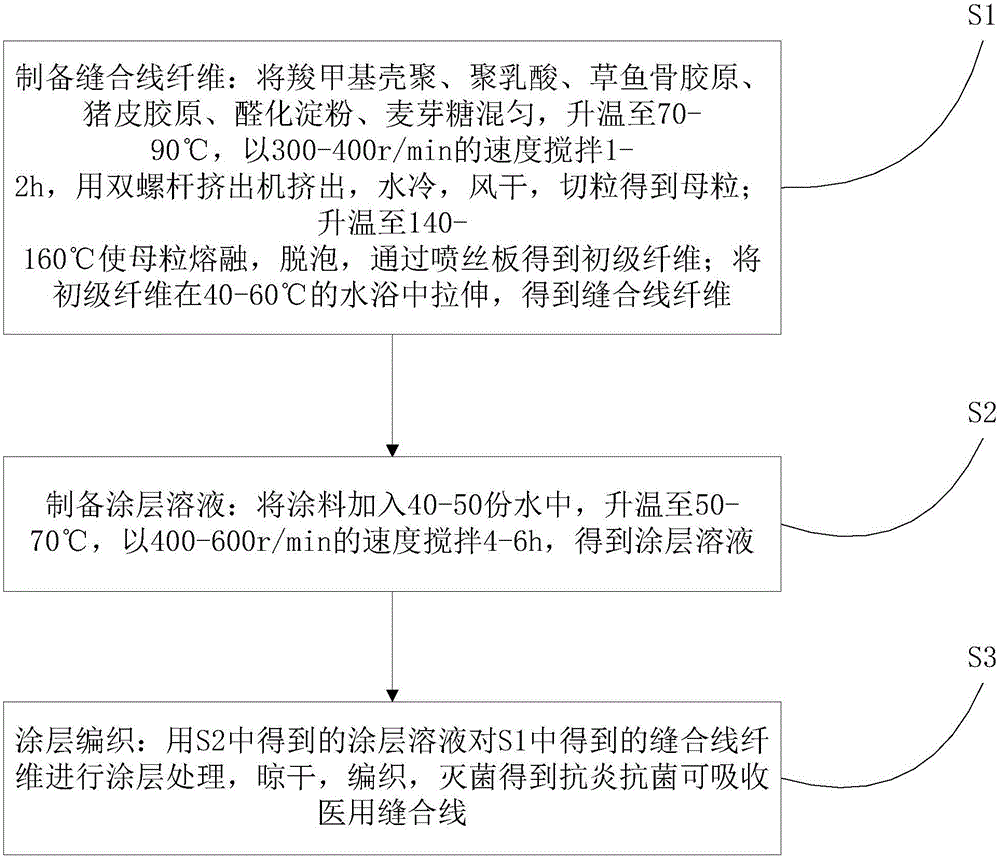
[0032] refer to figure 1 , the preparation method of a kind of anti-inflammatory and antibacterial absorbable medical suture that the present invention proposes, comprises the steps:
[0033] S1. Preparation of suture fiber: Mix carboxymethyl chitosan, polylactic acid, grass carp bone collagen, pigskin collagen, formaldehyde starch, and maltose, heat up to 70-90°C, and stir at a speed of 300-400r / min for 1 -2h, extrude with a twin-screw extruder, water-cooled, air-dried, and pelletized to obtain masterbatches; heat up to 140-160°C to melt the masterbatch, defoam, and obtain primary fibers through a spinneret; primary fibers at 40- Stretching in a water bath at 60°C to obtain suture fibers;
[0034] S2. Prepare a coating solution: add the coating to 40-50 parts of water, heat up to 50-70° C., and stir at a speed of 400-600 r / min for 4-6 hours to obtain a coating solution;
[0035] S3. Coating weaving: coating the suture fiber obtained in S1 with the coating solution obtained ...
Embodiment 1
[0038] An anti-inflammatory and antibacterial absorbable medical suture, its raw materials include by weight: 80 parts of carboxymethyl chitosan, 20 parts of polylactic acid, 10 parts of grass carp bone collagen, 12 parts of pig skin collagen, 4 parts of formaldehyde starch, 4 parts maltose, 1 part paint;
[0039] Among them, the preparation method of the coating is as follows: add chitosan to water, disperse evenly by ultrasonic, add fumaric acid and mix well, add perchloric acid dropwise, heat up to 70°C, keep stirring for 5 hours, and obtain solution A; use NaHCO 3The solution adjusts the pH of solution A to 8, adds it dropwise into acetone, filters, washes twice with ethanol, filters, adds ethanol, Soxhlet extraction for 60 hours, and vacuum-dries to obtain modified chitosan, wherein, chitosan and water The weight to volume ratio (g / ml) of chitosan and fumaric acid is 1:60, the weight to volume ratio of chitosan to fumaric acid is 1.7:5.5, and the weight to volume (g / ml) r...
Embodiment 2
[0045] An anti-inflammatory and antibacterial absorbable medical suture, its raw materials include by weight: 100 parts of carboxymethyl chitosan, 10 parts of polylactic acid, 15 parts of grass carp bone collagen, 8 parts of pig skin collagen, 6 parts of formaldehyde starch, 2 parts maltose, 5 parts paint;
[0046] Among them, the preparation method of the coating is: adding chitosan to water, ultrasonically dispersing evenly, adding fumaric acid and mixing, adding perchloric acid dropwise, raising the temperature to 50°C, and stirring for 7 hours to obtain solution A; using NaHCO 3 The solution was adjusted to pH=6 of solution A, added dropwise to acetone, filtered, washed 4 times with ethanol, filtered, added with ethanol, extracted by Soxhlet for 48 hours, and dried in vacuum to obtain modified chitosan, wherein chitosan and water The weight-to-volume ratio (g / ml) of chitosan and fumaric acid is 1:80, the weight-to-volume ratio (g / ml) of chitosan to perchloric acid is 1.7:5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com