RANKL-TNF sample region mouse monoclonal antibody and its preparation method and use
A monoclonal antibody and mouse-derived technology, applied in biochemical equipment and methods, antibodies, and microbial-based methods, can solve the problems of high price, increased pain and economic pressure of RA patients, and achieve inhibition of differentiation and alleviation of bone loss , the effect of mitigating progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 animal immunization
[0031] RANKL-TNF fusion protein immunized 6-8w female BALB / c mice (SPF grade), divided into protein immunization group and control group according to body weight, and subcutaneously immunized mice at multiple points in the abdomen (recorded as the 0th day after the first immunization, respectively on 21d, 21d, 42d, 63d booster immunization), each mouse immunization dose is 100μg (200μl). For the initial immunization, the immunogen was emulsified with an equal amount of Complete Freud’s adjuvant (CFA), and for booster immunization, the immunogen was emulsified with an equal amount of Incomplete Freud’s adjuvant (Incomplete Freud’s adjuvant, IFA). One week after each immunization, blood was collected from the orbital venous plexus, the serum was separated, and the level of neutralizing antibodies produced in the mice was detected by indirect ELISA. The specific operation is to coat the ELISA plate with RANKL-TNF fusion protein (5 μg / ml)...
Embodiment 2
[0032] Example 2 Preparation of RANKL-TNF-like region monoclonal antibody
[0033] On the third day after the shock immunization, the mouse with the highest antibody titer was selected, sacrificed by cervical dislocation, the spleen of the mouse was removed under a sterile environment, the connective tissue was separated, the spleen was cut into pieces, and the spleen cells were obtained by grinding with a 200-mesh cell grinder. liquid. The myeloma cell line SP2 / 0 was resuscitated one week in advance and cultured in RPMI1640 medium containing 20% fetal bovine serum (FBS). After harvesting the myeloma cells, count the myeloma cells and splenocytes respectively, mix the splenocytes and myeloma cells at a ratio of 5:1, centrifuge at 4°C, 1000rpm / min, 10min, remove the cell supernatant, flick At the bottom of the centrifuge tube, break up the cell pellet, add 1ml of 50% MW4000 polyethylene glycol (PEG) drop by drop while stirring and mixing within 1min, then stir the cell pelle...
Embodiment 3
[0034] Example 3 Subclonal Screening and Identification of Monoclonal Cell Lines
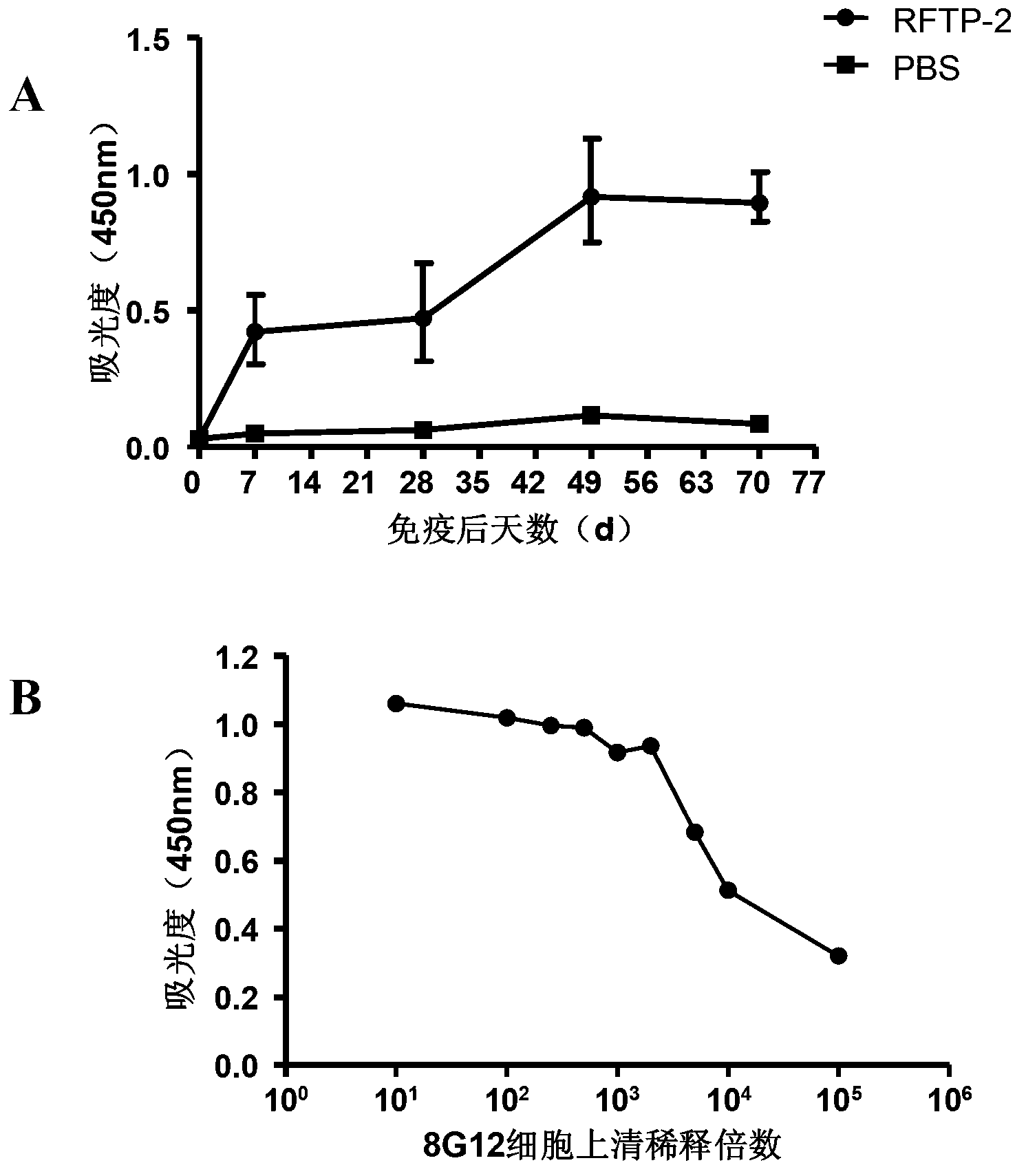
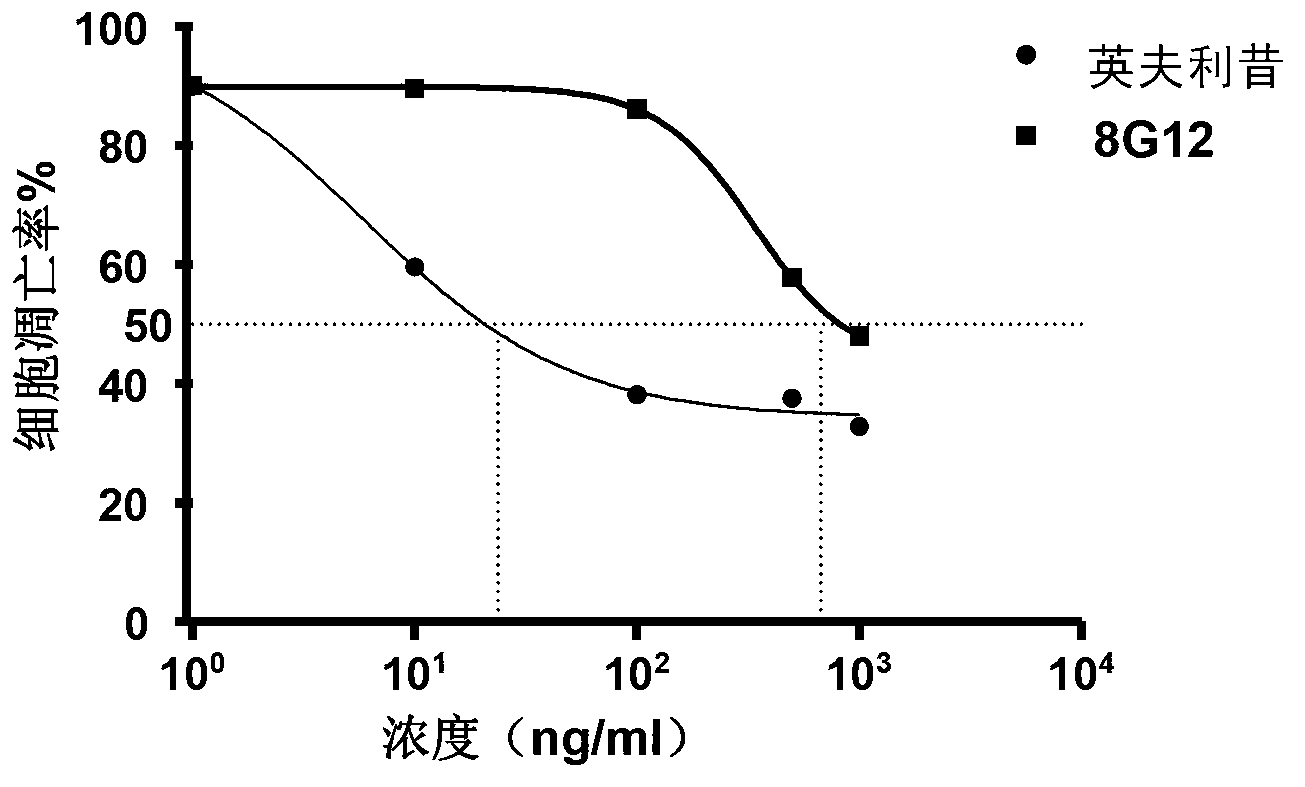
[0035] 5-7 days after cell fusion, observe the state of the cells in each well under the microscope. The clear cells arranged in piles under the microscope are the successfully fused hybridoma cells. Coat the ELISA plate with RANKL-TNF fusion protein (5 μg / ml), overnight at 4°C, and block with 5% PBST skimmed milk powder at room temperature for 4 hours, take 50 μl of the cell supernatant for indirect ELISA detection, and dilute the HRP-labeled secondary antibody at 1:20,000. Select to produce high-titer antibodies (such as figure 1 The titer shown in B is 10 4 ) cells were subcloned.
[0036] Mouse peritoneal macrophages were prepared the day before subcloning as feeder cells. The specific method is as follows: after 6-8w BALB / c (SPF grade) cervical dislocation, 75% ethanol soaked and disinfected, cut open the abdominal skin and peritoneum, clamped the peritoneum with tweezers, cut a small mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com