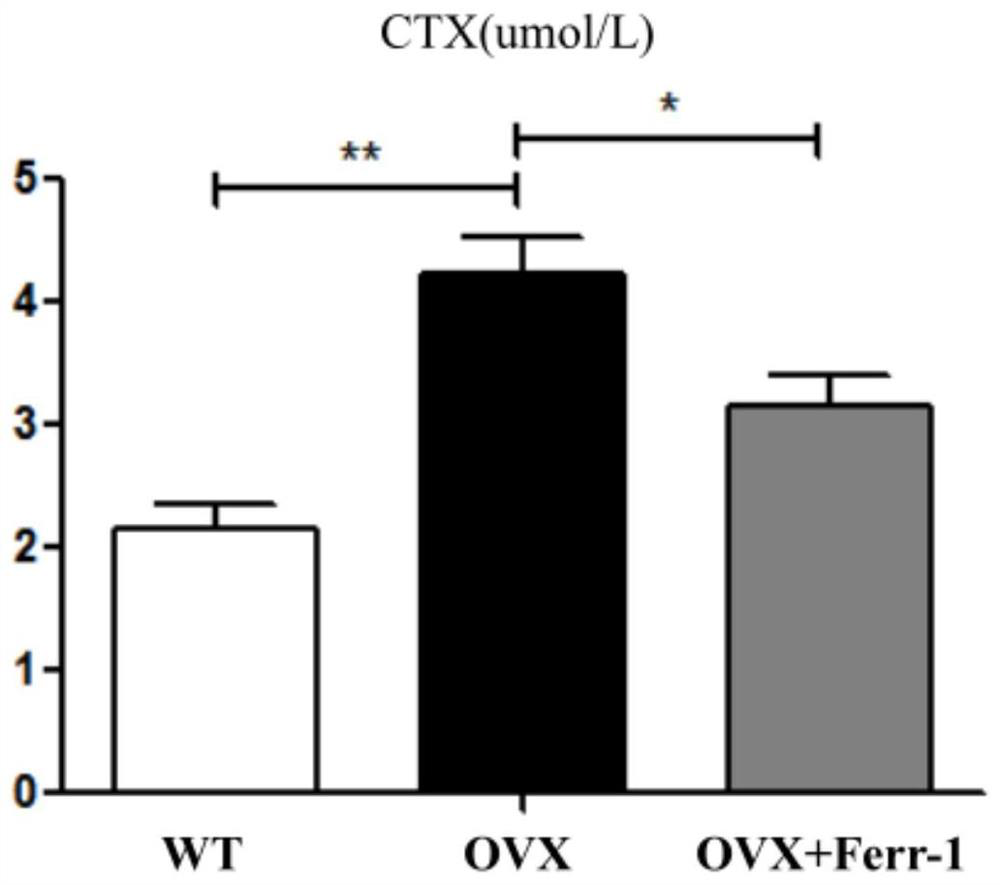
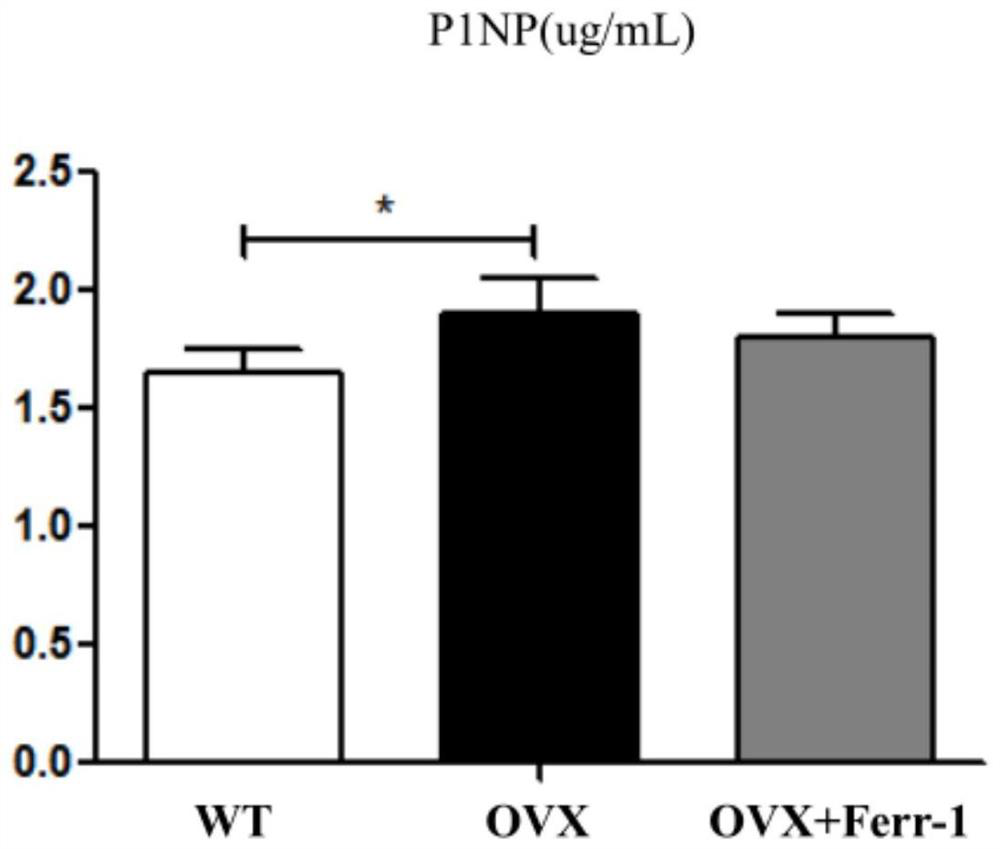
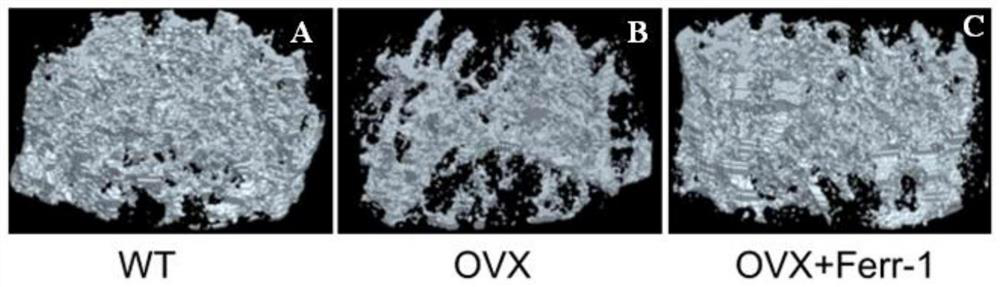
Use of ferrostatin-1 in the treatment of osteoporosis
A technology for osteoporosis and disease, applied in the field of medicine, can solve problems such as complex development mechanism and incomplete curative effect, achieve the effect of less dosage, expand the application field of disease, and have less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0025] 1. Materials
[0026] 1. Experimental animals
[0027] C57 / B6J mice, 15, female, 20 weeks old, weighing 21-37 g, SPF grade, provided by Shanghai Slack Laboratory Animal Co., Ltd. Laboratory animal production license number (SCXK (Shanghai) 2012-0002).
[0028] During the experiment, the temperature of the rearing room was 20±3.2°C, the relative humidity was 65-75%, and the animals had free access to food and water.
[0029] 2. Drugs and reagents:
[0030] 2.1 Drugs
[0031] Sirolimus tablets, trade name Rapa Ming, tablets, 1 mg / tablet, provided by Wyeth Pharmaceuticals (China) Co., Ltd., stored in a refrigerator at 4°C.
[0032] 2.2 Reagents
[0033] Ferrostatin-1, white powder, purity ≥95%, 1 mg / bottle, provided by Sigma-Aldrich Company in the United States, product number is SML0583, stored in a refrigerator at 4°C in the dark.
[0034] β-CTX ELISA kit, provided by China DL Develop Donglin Technology Company, the product number is DL-bCTx-Mu-48T.
[0035] P1NP ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com