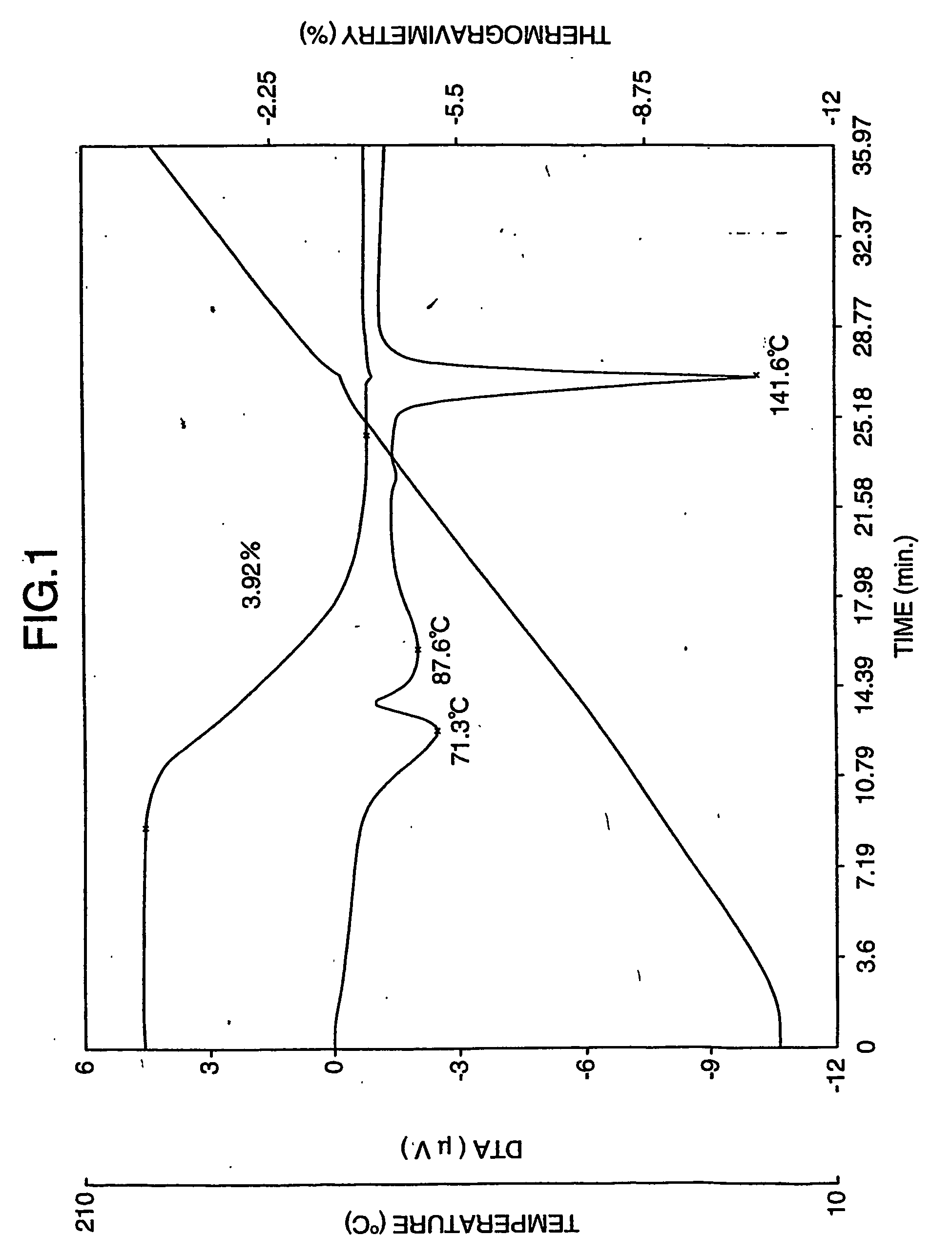
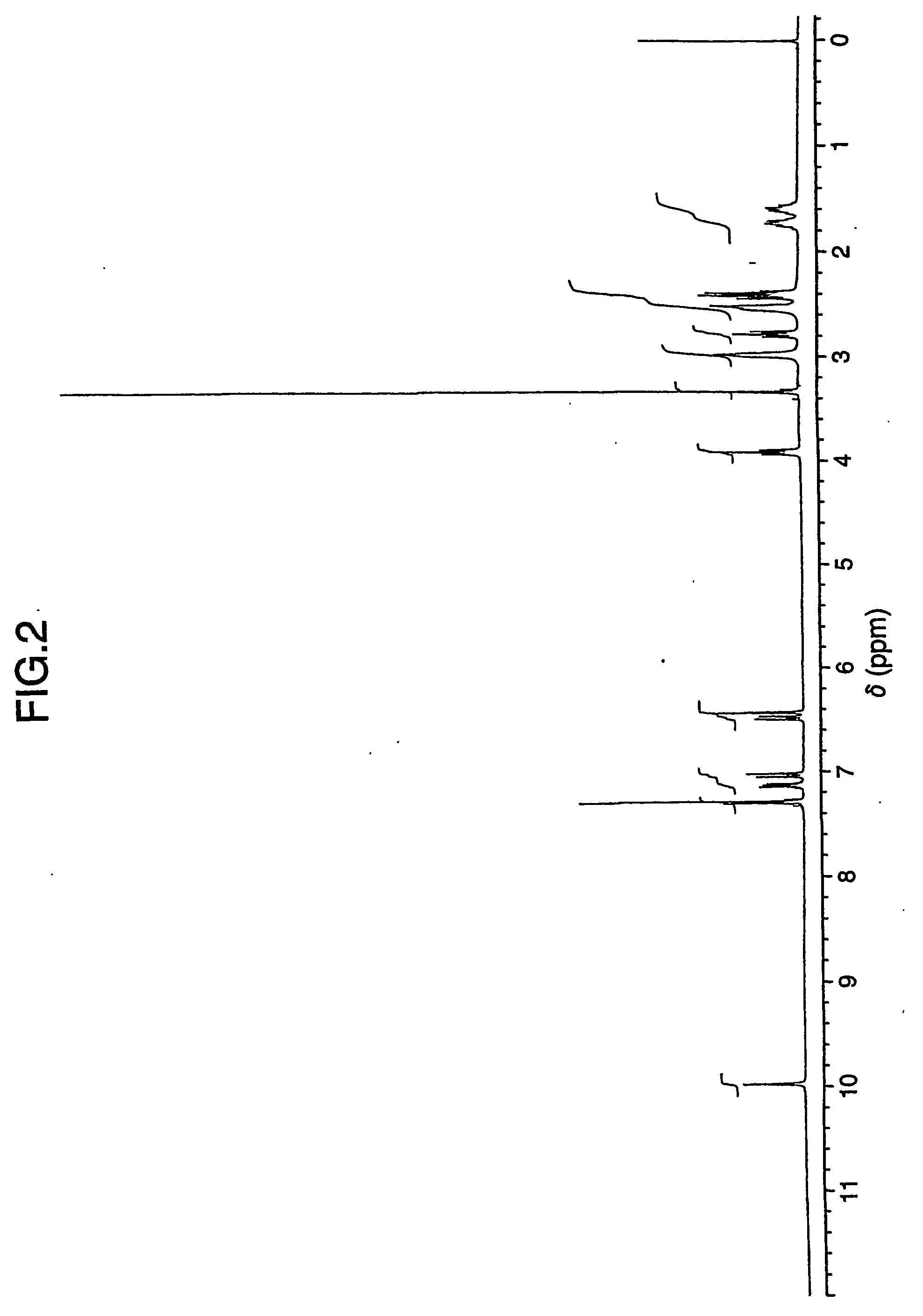
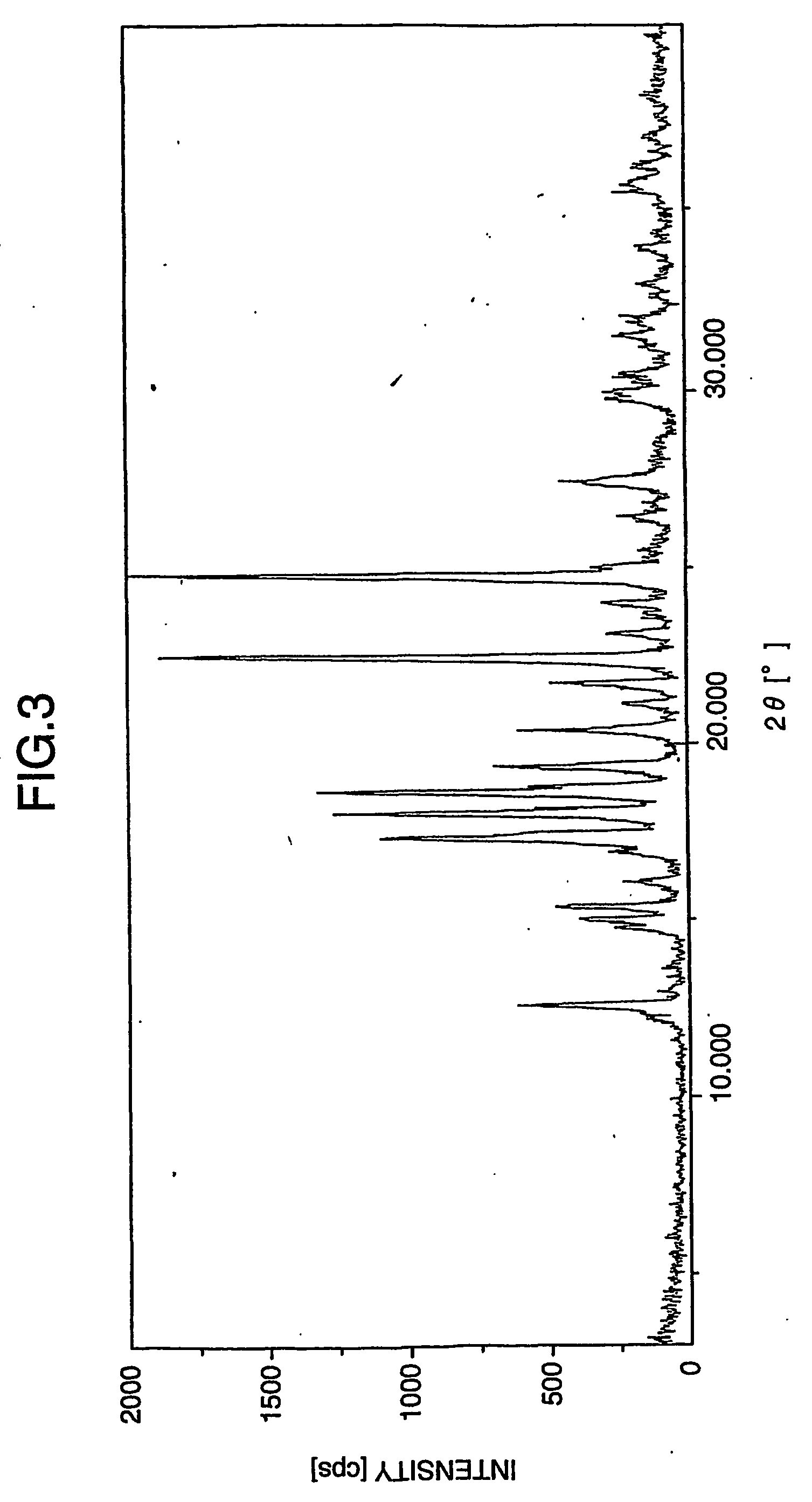
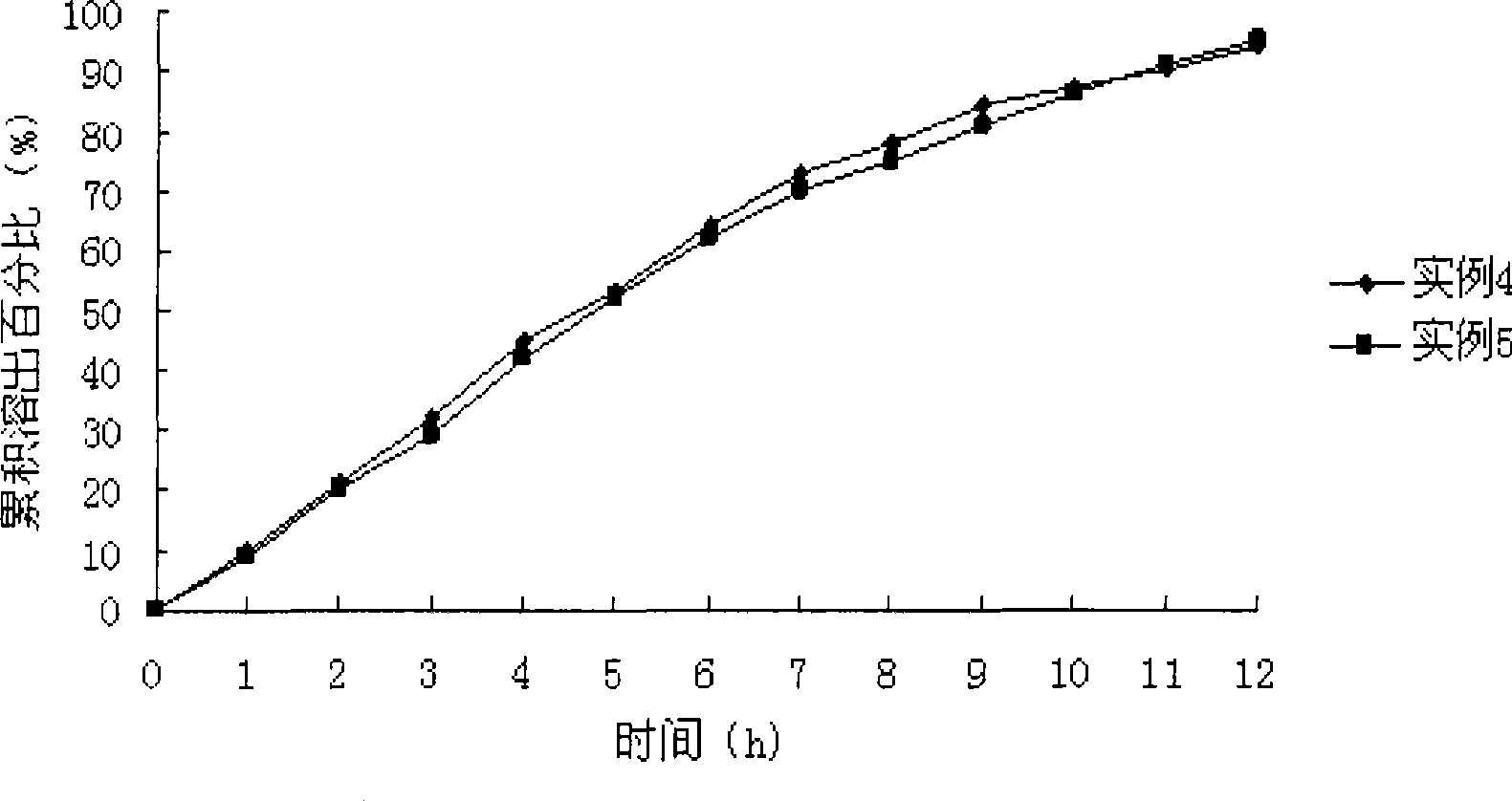
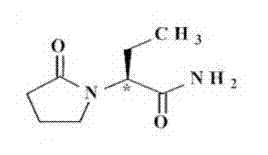
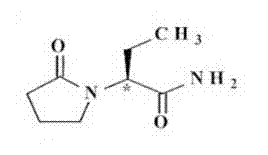
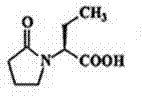
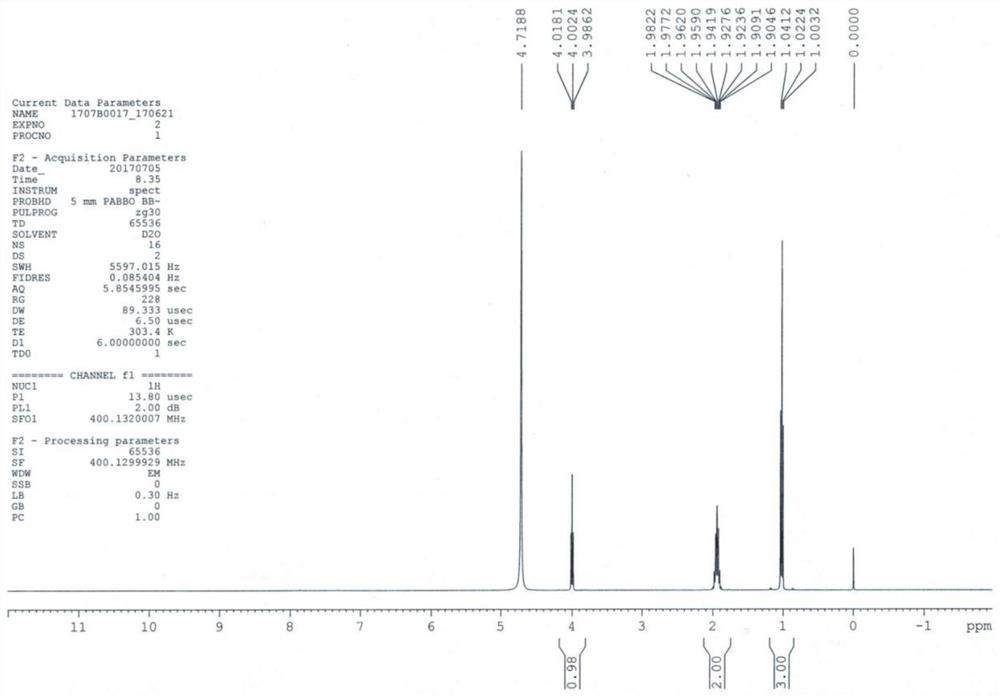
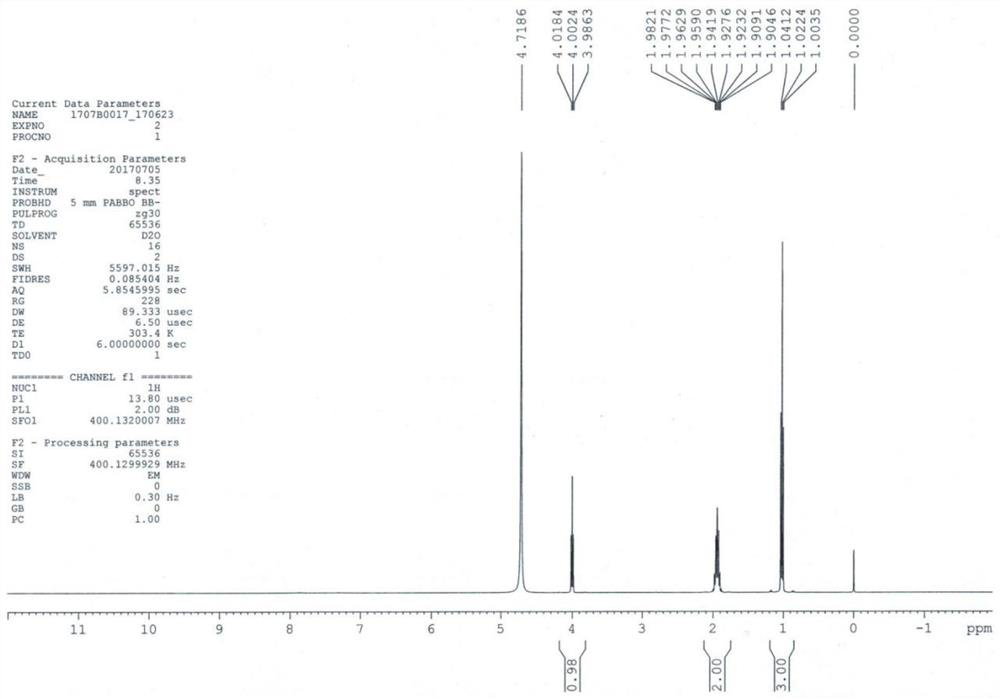
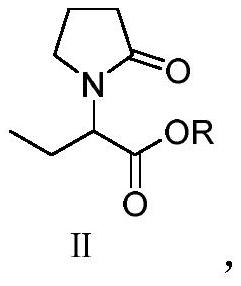
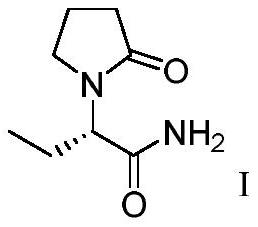
Patents
Literature
49 results about "Etiracetam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
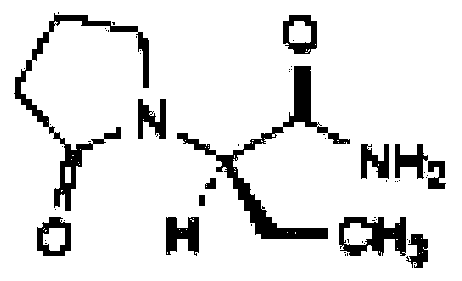
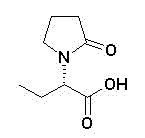
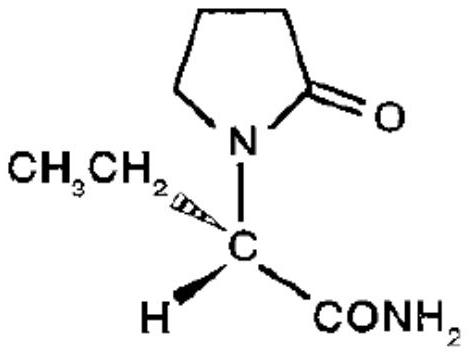
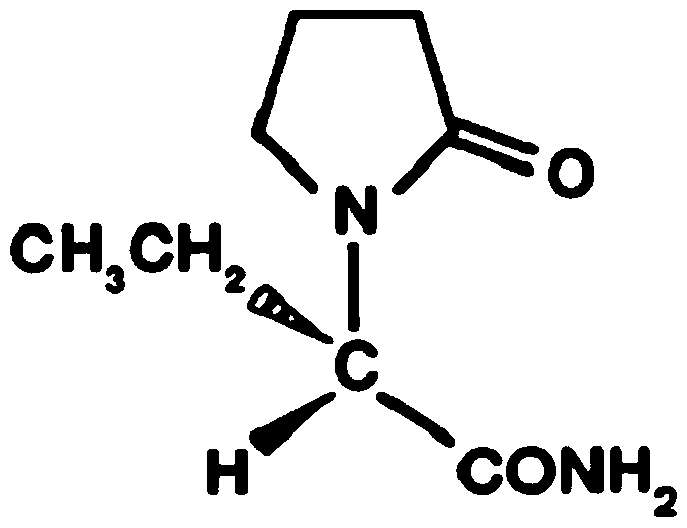
Etiracetam is a chemical compound belonging to the racetam family, which was developed as a nootropic drug. It is racemic; its biologically active enantiomeric form is levetiracetam, now marketed as an antiepileptic drug.
Carbostyril derivatives and mood stabilizers for treating mood disorders
The pharmaceutical composition of the present invention comprises a carbostyril derivative which is a dopamine-sero-tonin system stabilizer and a mood stabilizer in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof. The mood stabilizer may include but is not limited to lithium, valproic acid, divalproex sodium, carbamaza-pine, oxcarbamazapine, zonisamide, lamotragine, topiramate, gabapentin, levetiracetam or clonazepam. These compositions are used to treat patients with mood disorders, particularly bipolar disorder with or without psychotic features, mania or mixed episodes. Methods are provided for separate administration of a carbostyril derivative and a mood stabilizer to a patient with a mood disorder.
Owner:OTSUKA PHARM CO LTD
Rapid disperse dosage form containing levetiracetam
ActiveUS9339489B2Easy to manageOvercome disadvantagesPowder deliverySheet deliveryDiseaseBiochemistry
Owner:APRECIA PHARMA LLC
Levetiracetam injection and preparation method thereof
The invention discloses a levetiracetam injection and a preparation method thereof. The levetiracetam injection is combined by a levetiracetam material and a buffer solution of different salts. When pH value is 5.0-6.0, the liquid agent has the best stability and the optimal curative effect.
Owner:SHANGHAI CHENPON PHARMA TECH
Levetiracetam injection and preparation method thereof
ActiveCN103432070AAvoid lostLess quantityOrganic active ingredientsNervous disorderSodium acetateMedicine
The invention relates to a levetiracetam injection and a preparation method thereof. Specifically, the levetiracetam injection disclosed by the invention comprises the following components: levetiracetam, sodium chloride, sodium acetate buffer salt and injection water used as an injection solvent. The invention further relates to a method for preparing the levetiracetam injection. According to the invention, the levetiracetam injection disclosed by the invention is unexpectedly found to have good pharmaceutical properties.
Owner:四川鼎诺泰宸科技有限公司 +1
Stable levetiracetam injection
The invention relates to a stable levetiracetam injection. In particular, the levetiracetam injection provided by the invention comprises levetiracetam, an acidic material, an alkaline material and injection water serving as an injection solvent. The pH value of the levetiracetam injection provided by the invention is 4.5-6.5. The invention further relates to a method for preparing the levetiracetam injection. The fact that the levetiracetam injection provided by the invention has good pharmaceutical properties is unexpectedly found.
Owner:四川鼎诺泰宸科技有限公司 +1
Levetiracetam osmotic pump controlled release tablet and preparation method thereof
InactiveCN101422442ASmooth and sustained releaseReduce toxic and side effectsNervous disorderPharmaceutical delivery mechanismSide effectActive matter
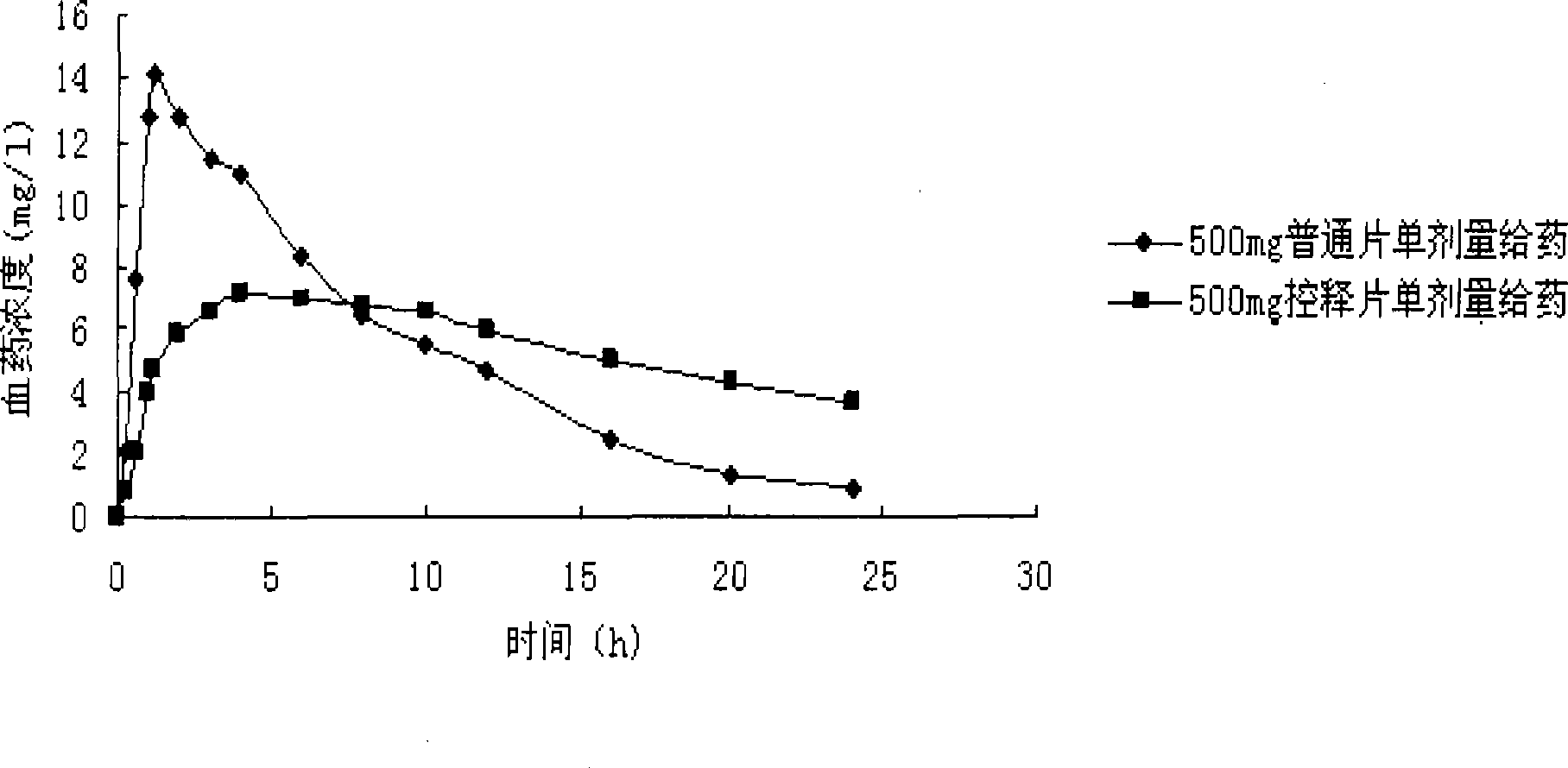
The invention belongs to the technical field of medicament and provides a Levetiracetam osmotic pump controlled-release tablet and a preparation method thereof. The invention consists of the accessory of the Levetiracetam playing the effect of release control and a semi-transparent membrane; in the invention, proper accessory and medicament are mixed to press a tablet core at first; then a layer of semi-transparent membrane is coated outside the tablet core; then at least one small hole is punched on the semi-transparent membrane so as to lead active matters to be released from the semi-transparent membrane, thereby controlling the release of the medicament. Compared with a common preparation, the controlled-release preparation prepared by the invention has the advantages of small wave range of the blood medicine concentration, reducing toxic and side effect, being taken once in one day and improving the compliance of sufferers. The controlled-release preparation is applied on the adjunctive therapy for the partial seizure of epileptics in clinic.
Owner:SHENYANG PHARMA UNIVERSITY
Levetiracetam injection
InactiveCN103690477AImprove stabilityReduce hidden dangersOrganic active ingredientsNervous disorderSodium acetateAcetic acid
The invention discloses a levetiracetam injection with a weight percent ratio of levetiracetam to cysteine of 10%:5%-10%; other auxiliary materials are sodium acetate, acetic acid, sodium chloride and injection water; the pH value of the injection is 5.0-6.5. The injection solves the problem that a process impurity of levetiracetam acid is increased slowly and the dosage is reduced during long-term storage of levetiracetam injections.
Owner:NANJING ZENKOM PHARMA
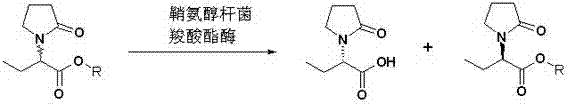
Sphingobacterium and method for preparing levetiracetam acid by utilizing same
InactiveCN102851238AMild reaction conditionsEasy to trainBacteriaMicroorganism based processesSphingobium chlorophenolicumHydrolysis
The invention discloses Sphingobacterium (sp.) SIT102 with the preservation number of CGMCC NO.6158 and a method for performing enantioselective hydrolysis to generate levetiracetam acid by utilizing the Sphingobacterium SIT102 serving as biocatalyst to catalyze racemization etiracetam acid esters. The method for preparing the levetiracetam acid comprises the steps of placing Sphingobacterium SIT102 cells into buffered solution, adding the racemization etiracetam acid esters into the buffered solution, performing the enantioselective hydrolysis through catalysis to obtain the levetiracetam acid. According to the method for preparing the levetiracetam acid by utilizing the Sphingobacterium SIT102 serving as the biocatalyst, the used biocatalyst is easy to prepare, the reaction condition is moderate, the yield of the levetiracetam acid can reach 48%, the enantiomer excess reaches 96%, and the production cost is low. Therefore, the method for preparing the levetiracetam acid by utilizing the Sphingobacterium has considerable industrial application development prospects.
Owner:SHANGHAI INST OF TECH
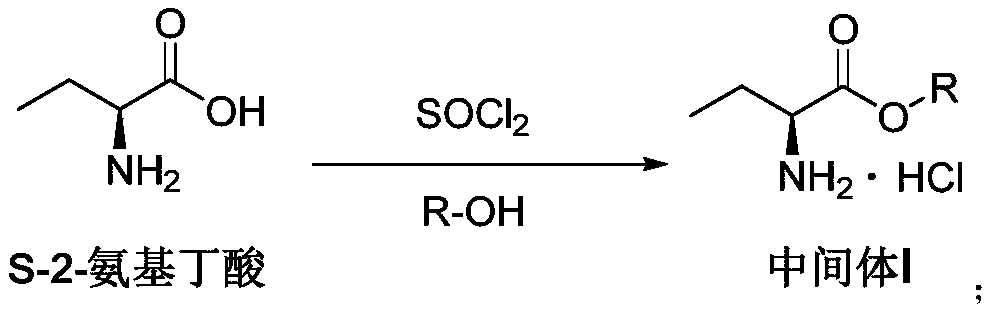
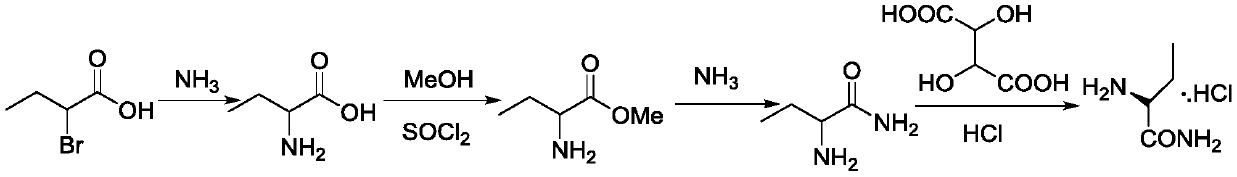
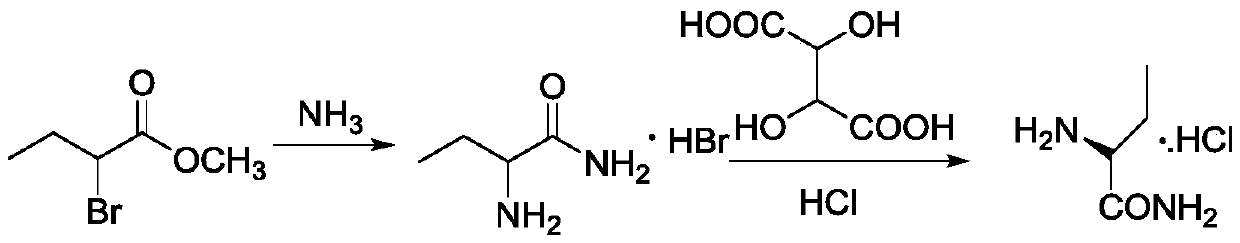
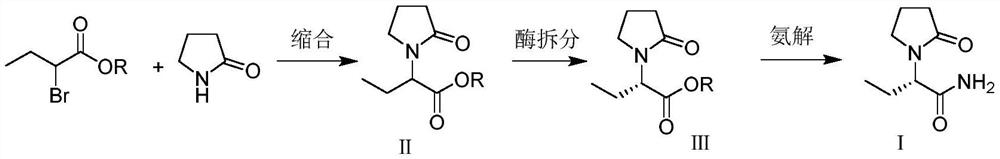
Synthesis, split and racemization method for preparing chirality medicament levetiracetam midbody (S)-(+)-2-amido butyramide hydrochlorate
ActiveCN101130504BReduce dosageHigh purityOrganic compound preparationCarboxylic acid amides optical isomer preparationEthyl groupRacemization
Owner:ABA CHEM CORP
Levetiracetam preparation method
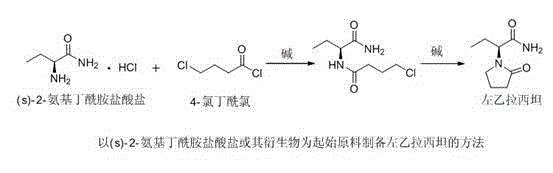
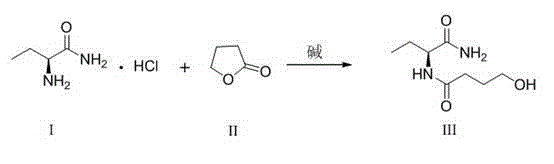
The present invention discloses a levetiracetam preparation method, wherein (s)-2-aminobutanamide and 1,4-butyrolactone are adopted as starting raw material, and two reactions such as aminolysis ring opening and acid-catalyzed dehydration cyclization are performed to prepare the levetiracetam. According to the present invention, the method has characteristics of low price of the used starting materials, environmental protection, high product yield, high product optical purity, simple reaction process, and no requirement of repeated chiral resolution; and the levetiracetam preparation method provides the new selection for the preparation and the production of the drug levetiracetam.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
Rapid disperse dosage form containing levetiracetam
A high dose rapidly dispersing three-dimensionally printed dosage form comprising a high dose of levetiracetam in a porous matrix that disperses in water within a period of less than about 10 seconds is disclosed. Also disclosed are methods of preparing the dosage form and of treating a condition, disease or disorder that is therapeutically responsive to levetiracetam. A process for preparing the dosage form is also provided
Owner:APRECIA PHARMA LLC
Levetiracetam sustained release pellets and preparation method thereof
InactiveCN104352446AReduce absorption rateStable absorptionOrganic active ingredientsNervous disorderSustained release pelletsAdhesive
The invention provides levetiracetam sustained release pellets. The levetiracetam sustained release pellets comprise medicine-containing pellets and coating layers, wherein the medicine-containing pellets are coated by the coating layers; the medicine-containing pellets comprise 250mg of levetiracetam, 70mg of hollow pellet cores, 60-110mg of filling agent, 18-68mg of lubricating agent and 2-10mg of adhesive; the coating layers comprise 45-225mg of Eudragit NE30D and 7-68mg of talcum powder. A preparation method of the levetiracetam sustained release pellets comprises the following processes: 1. material preparation; 2. mixing; 3. preparation of the adhesive; 4. preparation of the pellets; 5. preparation of a coating agent; 6. coating; 7. filling; 8. aluminium-plastic packaging and preparation of finished products. The levetiracetam sustained release pellets used for treating epilepsy and the preparation method have the beneficial effects that as the two kinds of advanced technologies, namely novel sustained release preparations and pellet preparations, are adopted, the levetiracetam sustained release pellets have stable treatment effects and higher bioavailability and have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; the preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Levetiracetam sustained release tablet and preparation method thereof
The invention provides a levetiracetam sustained release tablet and a preparation method thereof. The preparation method provided by the invention is as below: drying raw materials and accessories for sustained release tablets; respectively weighing particles dividing into a blank layer (taking a release material and a binder according to the proportion of the blank layer, mixing uniformly according to an equal incremental method and sieving) and a sustained release layer (taking blending ratio of levetiracetam, a sustained-release material and a binder, according to the proportion of the release layer, mixing uniformly according to an equal incremental method and sieving); adding a lubricant; mixing uniformly; and pressing the mixture in two hopper of a bilayer tablet machine to obtain the tablets.
Owner:南京亿华药业有限公司
Fast Dispersing Dosage Form Containing Levetiracetam
The present invention discloses a high dose rapidly dispersing three-dimensional printing dosage form comprising a high dose of levetiracetam in a porous matrix that disperses in water in a time period of less than about 10 seconds. Also disclosed are methods of making the dosage forms and methods of treating diseases, conditions or disorders that are therapeutically responsive to levetiracetam. The invention also provides methods for preparing the dosage forms.
Owner:APRECIA PHARMA LLC
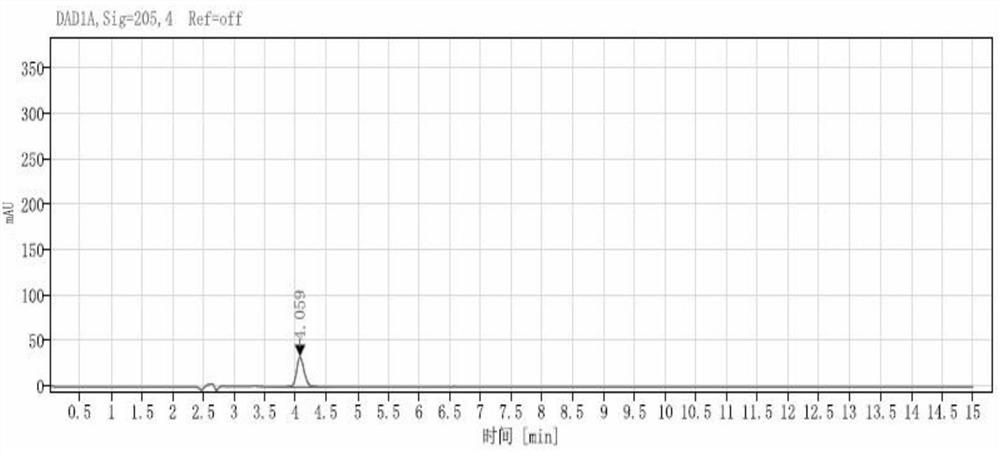
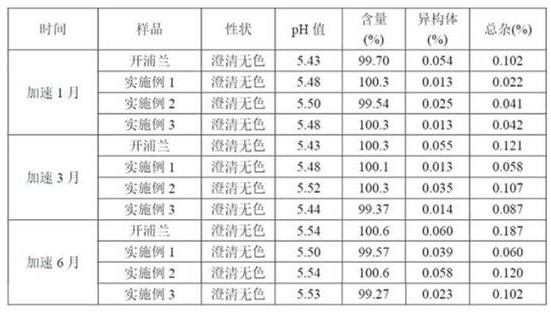
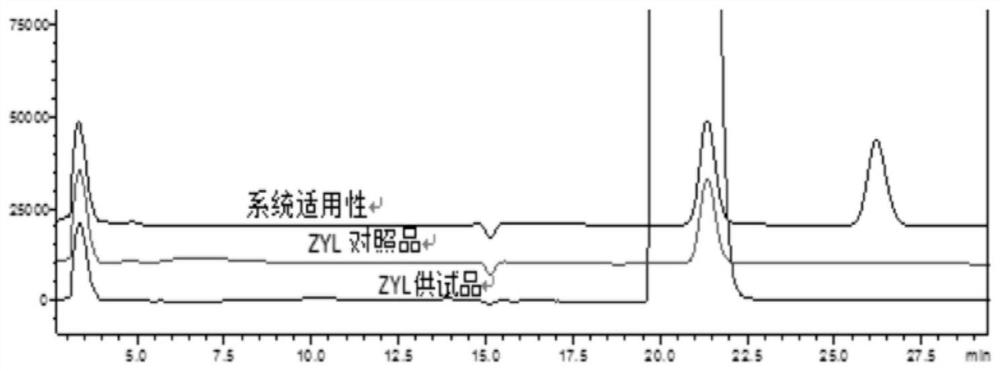
Method for detecting levetiracetam enantiomer in levetiracetam raw material or sodium chloride injection
The invention provides a method for detecting a levetiracetam enantiomer in a levetiracetam raw material or a sodium chloride injection, and belongs to the field of medicine quality detection. The detection method provided by the invention comprises the steps of diluting a levetiracetam raw material or a sodium chloride injection to obtain a test solution; and detecting the levetiracetam enantiomer in the test solution by adopting a high performance liquid chromatography, and calculating the content of the levetiracetam enantiomer in the levetiracetam sodium chloride injection according to a concentration-peak area standard curve of the levetiracetam enantiomer, wherein a chromatographic column of the high performance liquid chromatography is a chiral reversed phase chromatographic column,and the mobile phase is a phosphate buffer solution, and the pH value of the mobile phase is 5.0-6.0. The detection method provided by the invention can be used for detecting the content of the levetiracetam enantiomer in the levetiracetam sodium chloride injection, and is relatively high in accuracy.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +1
Levetiracetam 3D printing preparation and preparation method thereof
ActiveCN111840243AReduce drug dose varianceImprove medication experienceOrganic active ingredientsAdditive manufacturing apparatusEtiracetamBiomedical engineering
The invention belongs to the field of medicines and preparations, and relates to a levetiracetam 3D printing preparation and a preparation method thereof. The levetiracetam 3D printing preparation comprises levetiracetam accounting for 40-70% of the weight of the preparation, a filler, a disintegrating agent, a flavoring agent, a flow aid, an antioxidant and an adhesive, wherein the root mean square height (Sq) of the roughness characteristic value of the levetiracetam 3D printing preparation is not higher than 45 microns, the arithmetic mean height (Sa) is not higher than 40 microns, and themaximum height (Sz) is not higher than 350 microns. The preparation provided by the invention has excellent surface smoothness, can realize rapid drug release within a few seconds, can realize flexible adjustment of drug dosage, and greatly improves child medication compliance.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Levetiracetam sustained-release tablets and preparation method thereof
PendingCN111249246AThe content of medicinal ingredients is stableGood slow releaseOrganic active ingredientsNervous disorderProlonged-release tabletBiochemistry
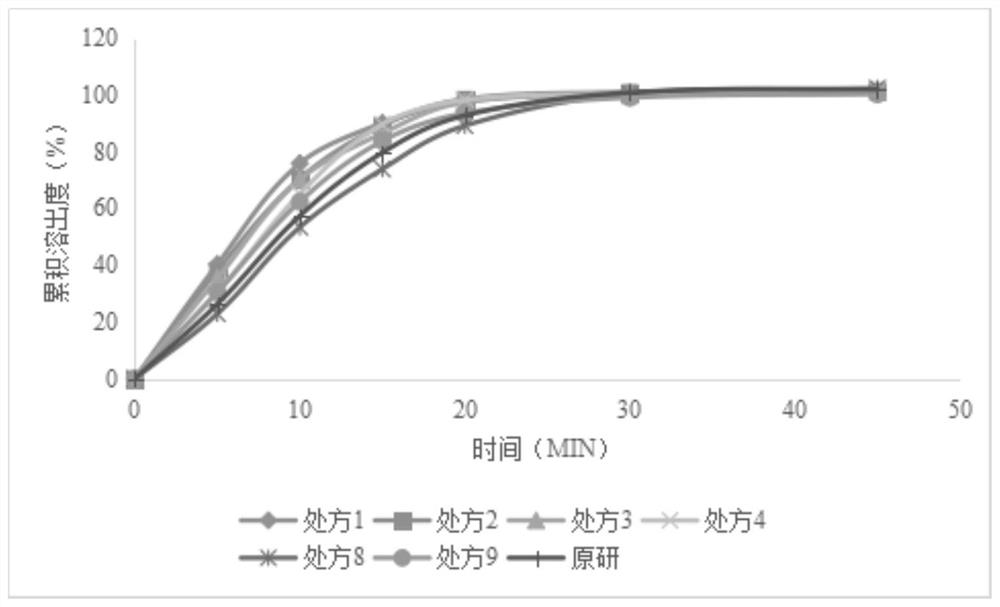
The invention discloses levetiracetam sustained-release tablets and a preparation method thereof, relates to the technical field of pharmaceutical preparations, and solves the problems that due to a poor release rate of levetiracetam sustained-release tablets, the actual pharmacological effect is greatly reduced, and the application effect is greatly reduced. The levetiracetam sustained-release tablets include the following components in percentages by weight: 45%-85% of levetiracetam, 1%-5% of a binder, 10%-50% of a sustained-release material, 0-2% of a flow aid, 0-2% of an anti-sticking agent, and 0-0.1% of a lubricant. The levetiracetam sustained-release tablets provided by the invention are easy to form as a whole, and have excellent release behavior and stable overall quality.
Owner:上海峰林生物科技有限公司
A kind of levetiracetam tablet and preparation method thereof
ActiveCN112870176BSimple processEasy to operateOrganic active ingredientsNervous disorderCellulosePowder mixture
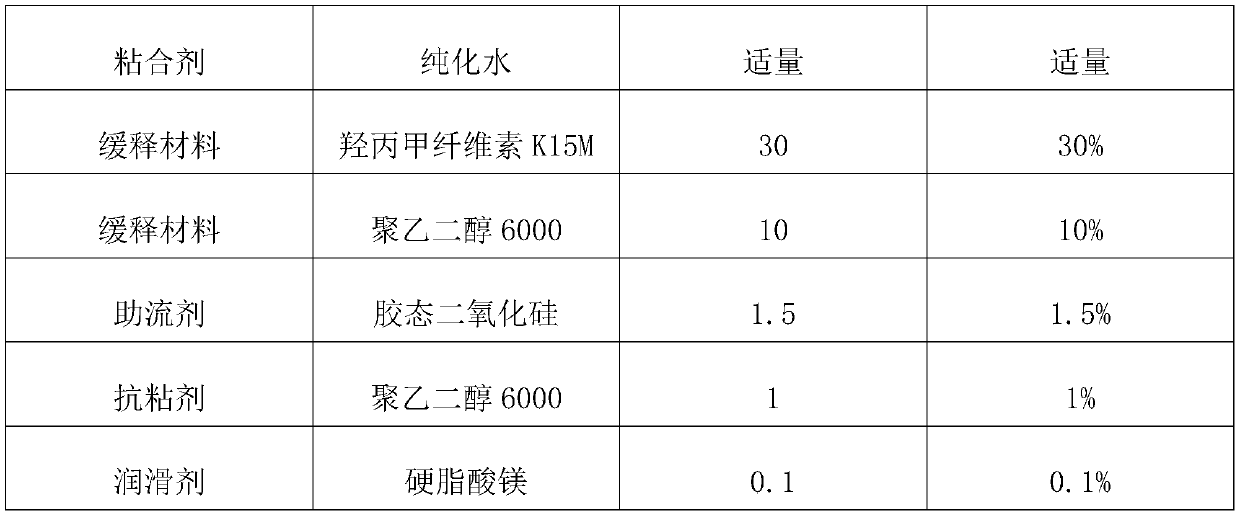
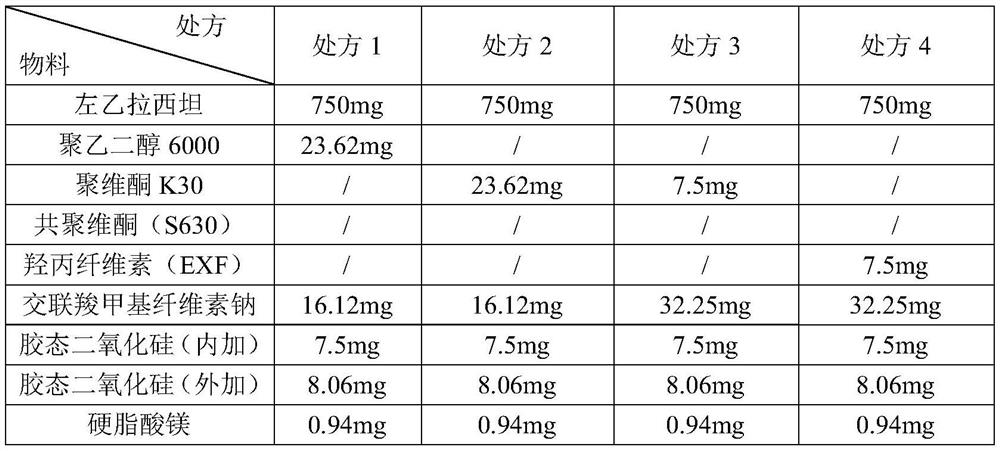
The invention belongs to the technical field of pharmaceutical preparations, in particular to a levetiracetam tablet and a preparation method thereof. The preparation method comprises the following steps: step 1: sieve levetiracetam, hydroxypropyl cellulose, croscarmellose sodium and part of colloidal silicon dioxide together for later use; step 2: sift the After the granulation materials are mixed, magnesium stearate is added and mixed evenly to obtain a premixed powder; step 3: the premixed powder in step 2 is placed in dry granulation and granulated to obtain a mixture of granules and powder; step 4: The mixture of granules and powder after the granulation in Step 3 is mixed with the remaining colloidal silicon dioxide and then compressed into tablets; Step 5: Coating. The preparation method provided by the invention has the advantages of simple technological process, strong operability, low production cost and stability.
Owner:HINYE PHARM CO LTD
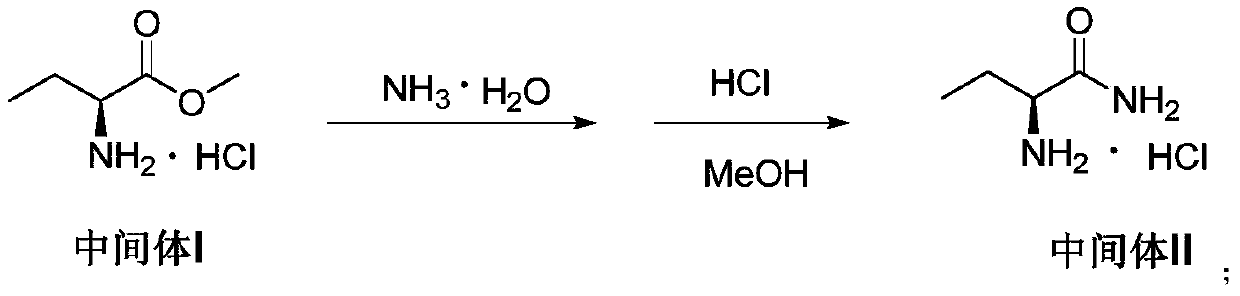
Method for preparing levetiracetam
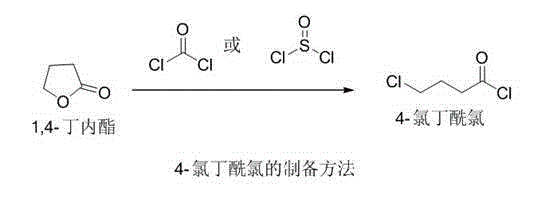
The invention relates to a method for preparing levetiracetam. The method comprises the following steps: reacting aminobutyric acid in lower alcohol and thionyl chloride to obtain an intermediate I; adding ammonia water to continue the reaction, and adding hydrochloric acid to adjust the pH value to about 3 to salify to obtain a salified intermediate II refined product; reacting the intermediate II in the presence of KOH in the presence of a catalyst and dichloromethane, and then adding 4-chlorobutyryl chloride to continuously react; adding water to hydrolyze, adjusting the pH to be weakly alkaline by using diluted hydrochloric acid, and crystallizing to obtain a levetiracetam crude product; decolorizing and crystallizing in ethyl acetate to obtain a refined product of levetiracetam. The invention also relates to the levetiracetam prepared by the method and pharmaceutical application thereof, for example, the levetiracetam can be used for treating or preventing epilepsy, Parkinson's disease, dyskinesia, migraine, tremor, idiopathic tremor, bipolar disorder, chronic pain, neuropathic pain, or bronchial, asthma or allergic diseases.
Owner:HUNAN DONGTING PHARMA
Preparation method of (S)-(+)-2-aminobutanamide hydrochloride
PendingCN111440083AHigh puritySimple and fast operationOrganic compound preparationCarboxylic acid amides preparationButyramideEsterification reaction
The invention discloses a preparation method of (S)-(+)-2-aminobutanamide hydrochloride, and relates to a preparation method of a levetiracetam key intermediate, and the method comprises the followingspecific steps: carrying out esterification reaction on L-2-aminobutyric acid serving as a starting material and thionyl chloride, and concentrating part of solvent after the reaction is finished; introducing ammonia gas to neutralize generated hydrogen chloride and residual thionyl chloride; and filtering, introducing ammonia gas, and carrying out an ammonolysis reaction to obtain the (S)-2-aminobutanamide hydrochloride after the treating is finished. According to the preparation method, the starting material is simple and easy to obtain, the one-pot method is adopted, the atom utilization rate is high, operation is easy and convenient, and the obtained product quality is high.
Owner:贵州阜康仁制药有限公司
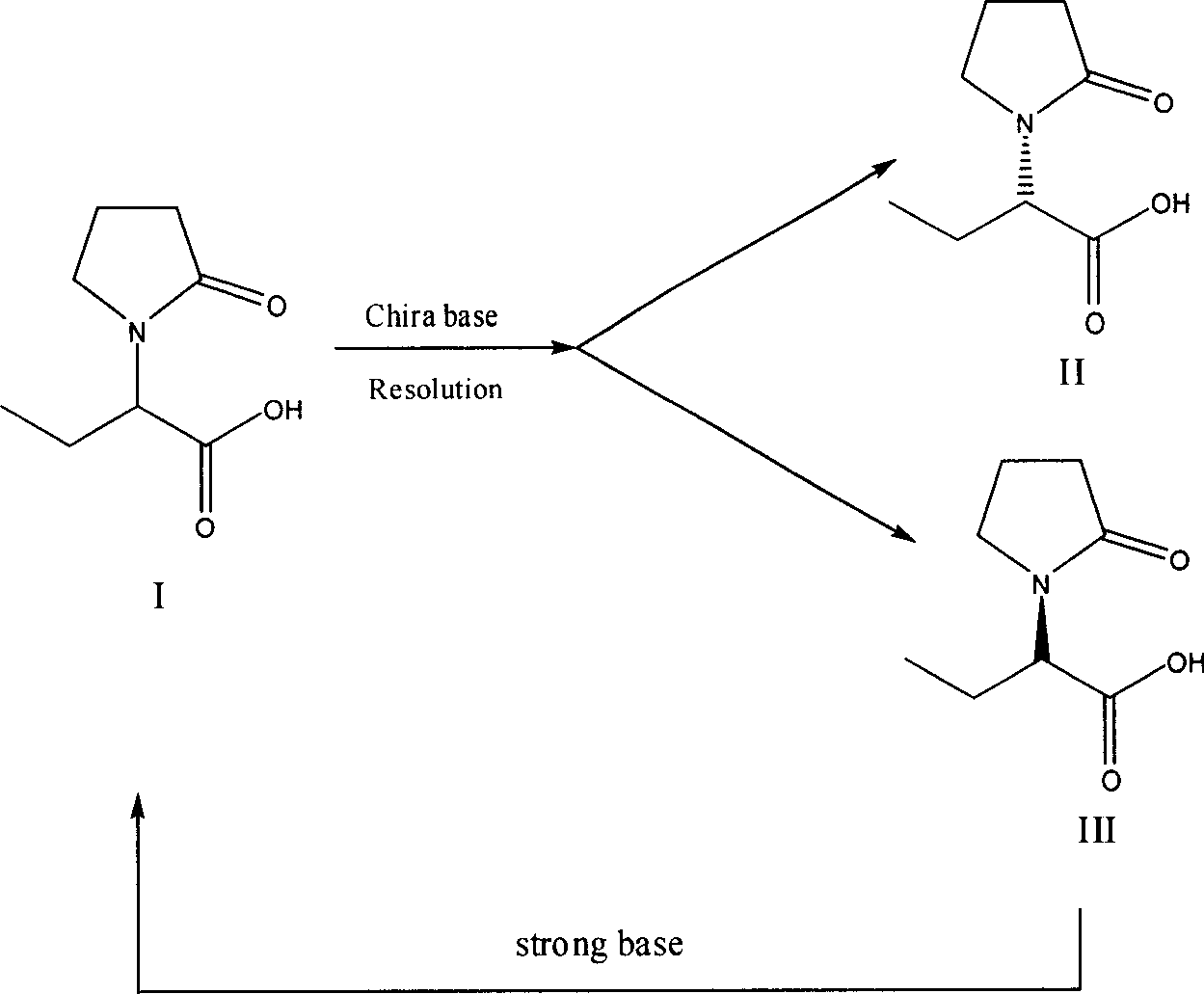
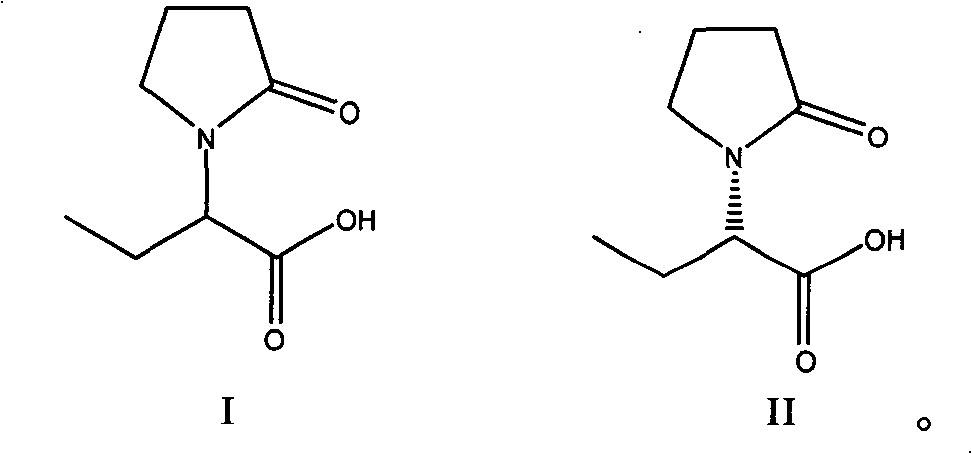
Method for preparing levetiracetam intermediate
ActiveCN101333180BHigh split efficiencyLow toxicityOptically-active compound separationOrganic racemisationAcetic acidAlcohol
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Pharmaceutical composition of levetiracetam and preparation method thereof
InactiveCN112263559AImprove stabilityReduce the number of handlingOrganic active ingredientsNervous disorderMedicinePharmaceutical drug
The invention relates to a pharmaceutical composition of levetiracetam and a preparation method thereof. The pharmaceutical composition comprises the following components in parts by weight: 0.3-6 parts of a disintegrating agent, 0.3-3 parts of an adhesive, 0.5-4 parts of a flow aid, 0.1-0.6 part of a lubricant and 92.6 parts of levetiracetam. The pharmaceutical composition of levetiracetam prepared by the invention has high stability; compared with the existing wet granulation and dry granulation, the disclosed preparation method has the characteristics of simple process, short operation time, low labor intensity and the like; and the prepared intermediate particles have good fluidity, and the pressed tablets are stable.
Owner:ZHEJIANG JIANGBEI PHARMA
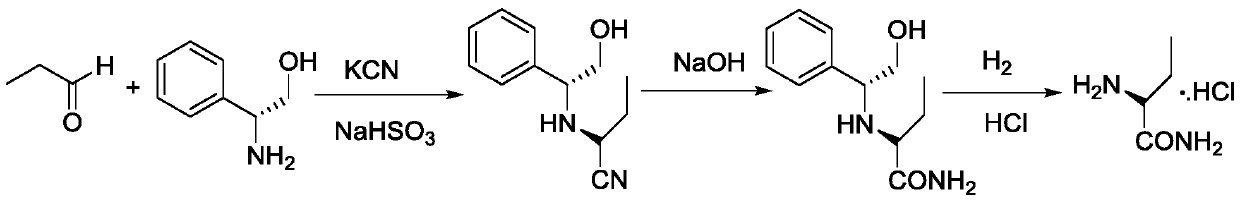
A kind of preparation method of levetiracetam key intermediate (s)-2-aminobutanamide salt
ActiveCN107673984BEasy to operateHigh response rateOrganic compound preparationOrganic chemistry methodsAlcoholButyramide
The invention discloses a preparation method of (S)-2-aminobutanamide as a key intermediate for levetiracetam, which belongs to the technical field of drug intermediate synthesis. According to the preparation method disclosed by the invention, a compound 1 undergoes ammonolysis reaction in C1-C3 alkyl alcohol, vacuum concentration is carried out until a dry state is formed after the reaction is complete, an alcoholic solvent is added, ammonia is further injected for freeing, an alcoholic solvent is added for clarification by dissolution after filtration and concentration, crystals are grown after an acidic alcoholic solvent is dripped for salification, a compound 2 is obtained by preparation and purification, wherein X is hydrochloric acid, hydrobromic acid or methanesulfonic acid. The preparation method disclosed by the invention is simple and effective, yield and purity are greatly increased, molar yield is higher than 90 percent, purity is higher than 99.5 percent, a high-quality intermediate is provided for the subsequent preparation of the levetiracetam, the preparation method does not have the step of chiral resolution, and adopts only one type of solvent, recovery is simple,three types of wastes are fewer, and the preparation method meets the requirement of industrial production.
Owner:ZHUHAI UNITED LAB
Preparation method of levetiracetam intermediate
The invention relates to a preparation method of alpha-ethyl-2-oxo-1-pyrrolidine acetate. Particularly, by optimizing a post-treatment process, the reaction yield and the product purity are remarkably improved.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +2
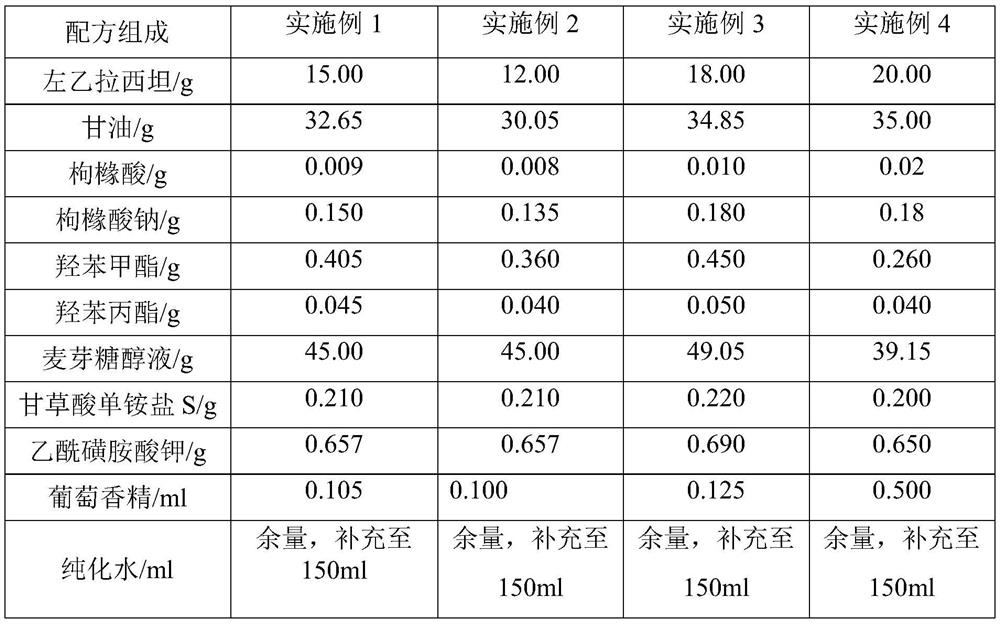
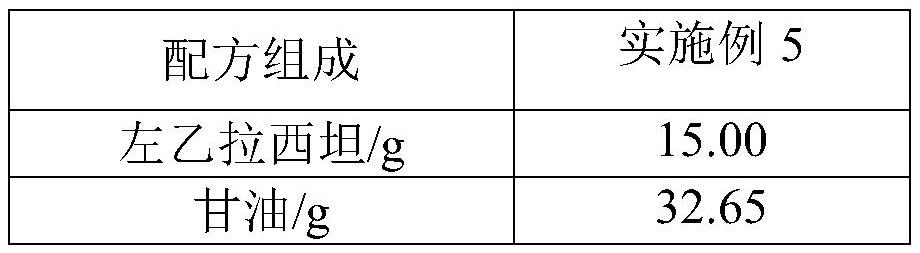
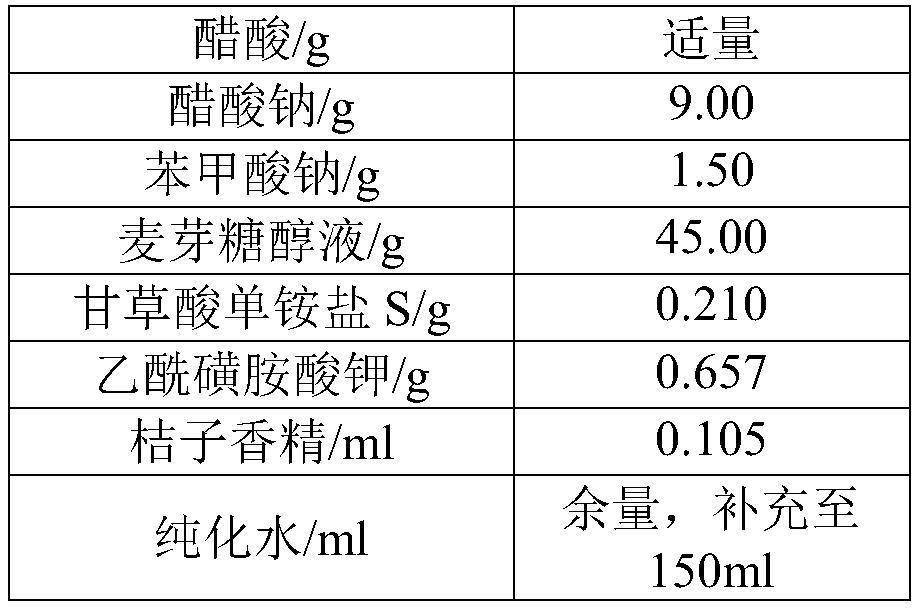
Levetiracetam oral solution and preparation method thereof
InactiveCN111759801AGreat tasteImprove clinical complianceOrganic active ingredientsNervous disorderAromatic agentBuffering agent
The invention belongs to the technical field of medicines, and particularly discloses a levetiracetam oral solution and a preparation method thereof. Each 150 ml of the levetiracetam oral solution comprises 12 to 20 g of levetiracetam, 0.1 to 0.2 g of a buffer agent, 0.3 to 0.5 g of a bacteriostatic agent, 45 to 50 g of a sweetening agent, 0.1 to 0.5 ml of an aromatic and the balance of a solvent.The preparation method comprises the following steps: dissolving the buffer agent in the solvent, heating the solvent to 70-80 DEG C, sequentially adding the bacteriostatic agent and the sweetening agent, performing uniform stirring, and keeping the temperature; cooling obtained mixture to 30-40 DEG C, adding the levetiracetam, performing stirring for dissolving, cooling stirred material to roomtemperature, adding the aromatic, performing uniform stirring, fixing the volume to full dose, performing uniform stirring, and performing filtration. The levetiracetam oral solution is proper in bacteriostatic agent content, good in bacteriostatic effect and high in safety; and in the preparation process, the increase of related substances of raw material medicines can be avoided through temperature rise and temperature reduction control.
Owner:HARBIN ZHENBAO PHARMA +1
A kind of levetiracetam sustained-release tablet and preparation method thereof
The invention provides a levetiracetam sustained-release tablet and a preparation method thereof. In the present invention, after drying the required raw materials and auxiliary materials of the sustained-release tablet, they are divided into a blank layer (according to the batching ratio of the blank layer, the slow-release material and adhesive are mixed uniformly and sieved) and the slow-release layer ( Take levetiracetam, sustained-release material and adhesive according to the batching ratio of the sustained-release layer, mix them uniformly according to the method of equal increase, and weigh the granules respectively, add lubricant, mix well, and place in a double-layer Tablets are made in the two hoppers of the tablet press.
Owner:南京亿华药业有限公司
Levetiracetam composition, preparation method and application
InactiveCN112190544AMeet the requirements of osmotic pressureReduce the drawbacks of difficult IV infusionOrganic active ingredientsNervous disorderAcetic acidIntravenous therapy
The invention belongs to the technical field of medicine, and discloses levetiracetam composition, a preparation method and an application. The levetiracetam composition contains levetiracetam, sodiumacetate trihydrate, sodium chloride, glacial acetic acid and water for injection, and the average osmotic pressure molar concentration of the composition is 291-304 mOsmol / kg. The mass part ratio ofthe levetiracetam to the sodium acetate trihydrate to the sodium chloride is (250-500): (1.5-4): (0.5-12). The pH value of the composition is 5.5 + / -0.3. Aiming at the defects that a levetiracetam preparation in the prior art cannot be directly subjected to intravenous injection and the compliance of a patient is poor, the invention provides the isotonic levetiracetam injection preparation which meets the osmotic pressure requirement of intravenous injection and can be directly subjected to intravenous injection without compatibility.
Owner:燃点(南京)生物医药科技有限公司
A method for detecting Devetiracetam from medicine
ActiveCN109765316BQuality improvementMeet impurity control requirementsComponent separationCelluloseCarbamate
The invention discloses a method for detecting devetiracetam (R-isomer) from levetiracetam injection, and adopts high performance liquid chromatography to carry out qualitative or / and quantitative detection of the R-isomer, The detection condition of liquid chromatography comprises: chromatographic column: cellulose-three (3,5-dichlorophenyl carbamate) silica gel column; Mobile phase: n-hexane, dehydrated alcohol; Wherein, n-hexane (vol.% ): absolute ethanol (vol.%)=90~70: 10~30; the mobile phase adopts isocratic elution. The method of the invention can effectively detect the levetiracetam in the levetiracetam injection, and has specificity and stability indicating ability. Moreover, the levetiracetam injection can be directly diluted and tested without pretreatment of samples, which is convenient and fast; the detection limit of the method of the invention reaches 0.003%, and the quantification limit reaches 0.005%, which is accurate and sensitive.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
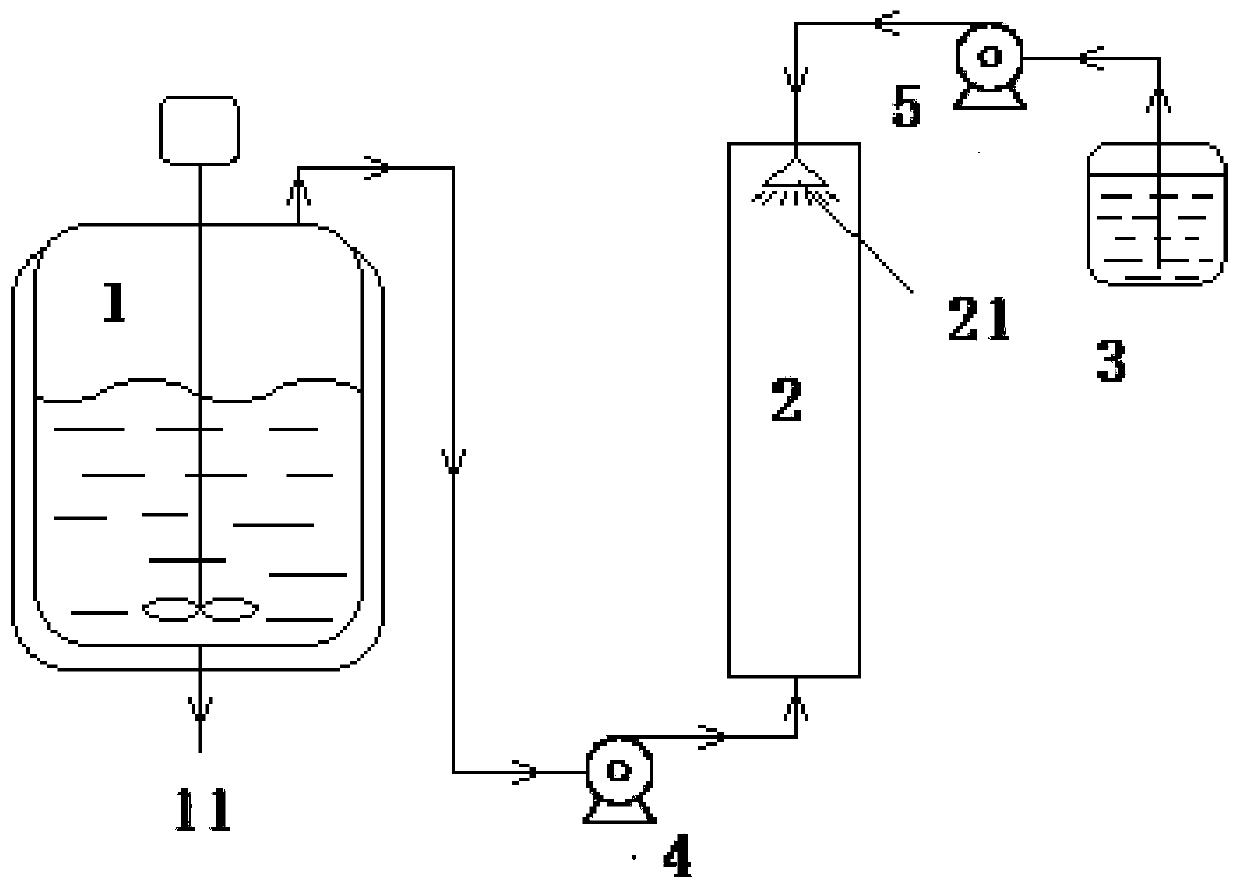
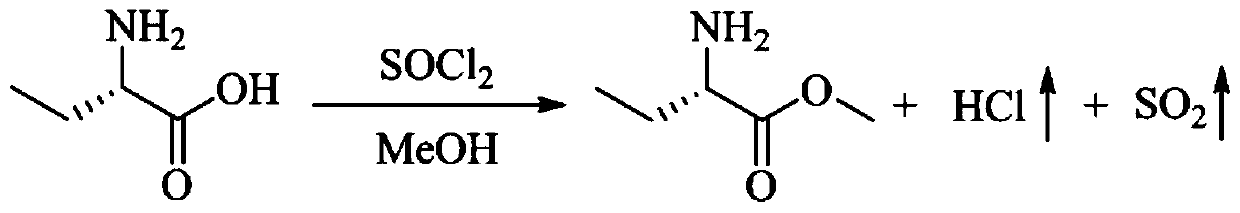
Green production method and device of levetiracetam key intermediate S-2-methyl aminobutyrate
PendingCN111004138AEffective absorptionProcess environmental protectionOrganic compound preparationOrganic chemistry methodsMethyl asterrateDistillation
The invention discloses a green production method of a levetiracetam key intermediate S-2-methyl aminobutyrate, and belongs to the field of medicine preparation, the method comprises the following steps: S1, pumping a mixture of S-2-aminobutyric acid, methanol and thionyl chloride into a reaction kettle, heating and stirring for reaction, and extracting the hydrogen chloride and the sulfur dioxidegenerated by reaction from the top of the reaction kettle; s2, pumping the hydrogen chloride and the sulfur dioxide extracted from the top of the reaction kettle into the bottom of an absorption tower, and pumping an alkaline aqueous solution into the tower top to absorb the hydrogen chloride and sulfur dioxide gas; and S3, discharging the reaction liquid from the bottom of the reaction kettle, and carrying out atmospheric distillation to obtain the S-2-methyl aminobutyrate. According to the process method provided by the invention, the hydrogen chloride and the sulfur dioxide generated in the production process of the S-2- methyl aminobutyrate are effectively absorbed, so that the process is greener and more environment-friendly, and meanwhile, the conversion rate of the S-2-methyl aminobutyrate in the obtained reaction product is greater than 88%.
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com