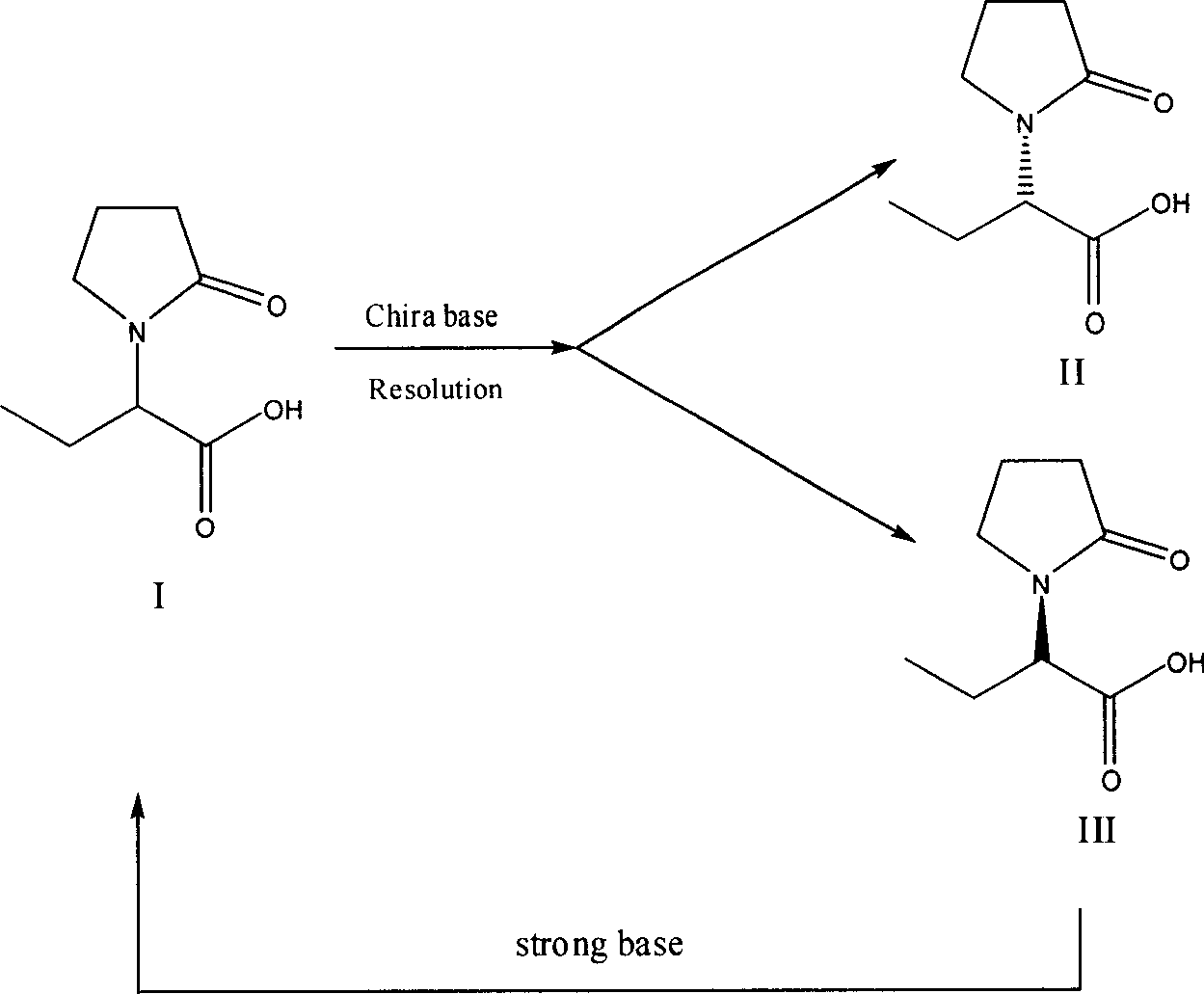
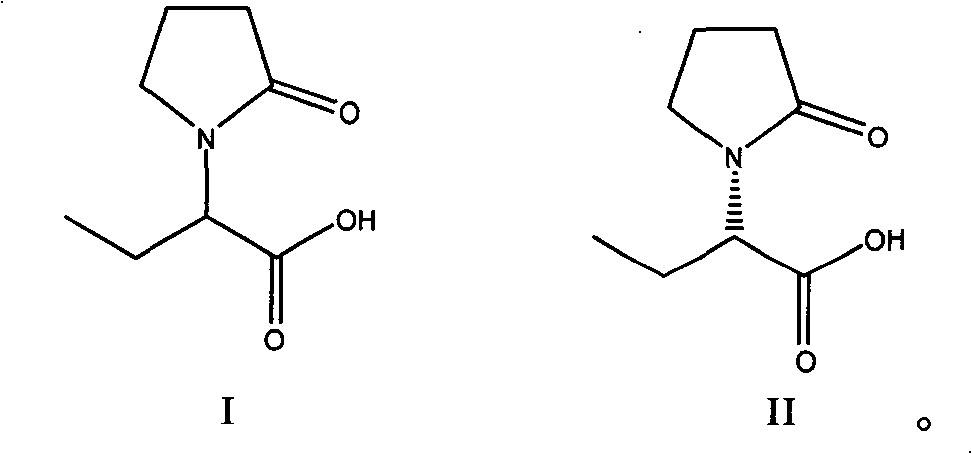
Method for preparing levetiracetam intermediate
An intermediate, ethyl technology, applied in the field of preparation of levetiracetam intermediate - α-ethyl-2-oxo-1-pyrrolidine acetic acid, can solve the problem of low optical purity and achieve low toxicity , the effect of high splitting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid (R)-α-methylbenzylamine salt
[0037] Put 26.4g of compound I and 80ml of acetone into a 250ml three-necked flask. Stirring was started, and a mixed solution of 9.33 g of (R)-α-methylbenzylamine and 7.83 g of triethylamine was added dropwise. After dropping, the temperature was raised to 50-60°C and refluxed for 2 hours. Remove insoluble matter by hot filtration, and the filtrate is cooled to 10-15° C., stirred and crystallized for 2 hours, filtered, the filtrate is the mother liquor to be recovered, the filter cake is washed with 10 ml of acetone, and dried to obtain 17 g.
[0038] Yield: 75.6% [α] 20 D =-18.5° (C=1, isopropanol)
[0039] Purification of (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid (R)-α-methylbenzylamine salt
[0040] Put 17g of (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid (R)-α-methylbenzylamine crude product into a 250ml three-necked flask, add 85ml of acetone, heat and reflux for 2 hours, a...
Embodiment 2
[0045] Preparation of (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid (R)-α-methylbenzylamine salt
[0046] 20g of compound I and 50ml of acetone were dropped into a 250ml three-necked flask. Stirring was started, and a mixed solution of 7 g of (R)-α-methylbenzylamine and 5.92 g of triethylamine was added dropwise. After dropping, the temperature was raised to 50-60°C and refluxed for 2 hours. Heat filtration to remove insoluble matter, the filtrate was cooled to 10-15°C, stirred and crystallized for 2 hours, filtered, the filtrate was the mother liquor to be recovered, the filter cake was washed with 10ml of acetone, and dried to obtain 11.7g.
[0047] Yield: 68.5% [α] 20 D =-20.5° (C=1, isopropanol)
[0048] Preparation of (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid
[0049] 11.7g (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid (R)-α-methylbenzylamine salt ([α] 20 D =-20.5° (C=1, isopropanol)) was dissolved in 20ml of water, temperature control T≤10°C, adjusted to PH=11-12 with 30...
Embodiment 3
[0051] Preparation of (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid (R)-α-methylbenzylamine salt
[0052] Put 20 g of compound I and 100 ml of ethyl acetate into a 250 ml three-necked flask. Stirring was started, and a mixed solution of 7 g of (R)-α-methylbenzylamine and 5.92 g of triethylamine was added dropwise. After dropping, the temperature was raised to 75-77°C and refluxed for 2 hours. Heat filtration to remove insoluble matter, the filtrate was cooled to 10-15° C. and stirred to crystallize for 2 hours, filtered, the filtrate was the mother liquor to be recovered, the filter cake was washed with 20 ml of ethyl acetate, and dried to obtain 14.4 g.
[0053] Yield: 84% [α] 20 D = -19° (C = 1, isopropanol)
[0054] Preparation of (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid
[0055] 14.4g (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid (R)-α-methylbenzylamine salt ([α] 20 D =-19 ° (C = 1, isopropanol)) was dissolved in 25.7ml of water, temperature control T ≤ 10 ° C with 30% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com