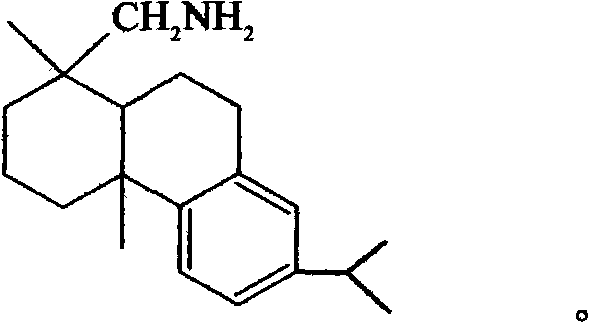
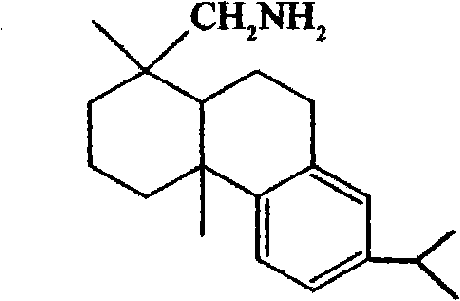
Resolving agent for 1, 1'-bi-2-naphthol and resolving method thereof
A resolving agent and technology of binaphthol, which is applied in the field of resolving agent and resolution of 1,1'-bin-2-naphthol, can solve the high cost of binaphthol resolution and unstable chemical properties , low splitting efficiency and other issues, to achieve the effect of high splitting efficiency, easy control of reaction conditions, and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 2.85 grams (0.01 mol) of optically active resolving agent (+)-dehydroabietylamine and 2.86 gram (0.01 mol) of racemic 1,1 '-linked-2-naphthol are dissolved in 30 milliliters of 80% ( V 甲醇 / V 水 ) in methanol aqueous solution, heated to reflux for 6 hours, cooled to -15°C, and white crystal S-(-)-binaphthol dehydroabietylamine salt was precipitated. After filtration, the mother liquor contains R-(+)-binaphthol dehydroabietylamine salt, which is collected for future use. The filter cake was treated with 30 milliliters of 5% potassium hydroxide aqueous solution, and the light yellow resolving agent (+)-dehydroabietylamine was separated out, and the (+)-dehydroabietylamine was extracted with 30 milliliters of toluene, and the toluene organic layer was collected for subsequent use. The water layer was neutralized to neutral with 5% sulfuric acid, and the white crystal S-(-)-binaphthol was precipitated, filtered, washed with distilled water, dried, and recrystallized with a ...
Embodiment 2
[0046] The mother liquor containing R-(+)-binaphthol dehydroabietylamine salt obtained in Example 1 was concentrated by distillation, and the methanol solvent was recovered. The distillation residue was treated with 30 ml of 5% potassium hydroxide aqueous solution, and the light yellow resolving agent (+)-dehydroabietylamine was separated out, and the (+)-dehydroabietylamine was extracted with 30 ml of toluene, and the toluene organic layer was collected for subsequent use , the water layer was neutralized to neutral with 5% sulfuric acid, and the white crystal R-(-)-binaphthol was precipitated, filtered, washed with distilled water, dried, and recrystallized with a small amount of toluene to obtain R-(+)- Binaphthol 1.02 g (yield 71%), melting point: 208 ° C ~ 210 ° C, [a] D 20 =+35.2° (C=1, THF), optical purity (ee value): 99.2%.
Embodiment 3
[0048] 5.70 g (0.02 mol) of optically active resolving agent (+)-dehydroabietylamine and 2.86 g (0.01 mol) of racemic 1,1'-linked-2-naphthol were dissolved in 40 ml of isopropyl In alcohol, heat to reflux for 1 hour, add 6 ml of water, cool to -10°C, and white crystal S-(-)-binaphthol dehydroabietylamine salt is precipitated. After filtration, the mother liquor contains R-(+)-binaphthol dehydroabietylamine salt, which is collected for future use. The filter cake was treated with 30 milliliters of 5% sodium hydroxide aqueous solution, and the light yellow resolving agent (+)-dehydroabietylamine was separated out, and the (+)-dehydroabietylamine was extracted with 30 milliliters of toluene, and the toluene organic layer was collected for subsequent use. The water layer was neutralized to neutral with 5% hydrochloric acid, and the white crystal S-(-)-binaphthol was precipitated, filtered, washed with distilled water, dried, and recrystallized with a small amount of toluene to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com