Industrial preparation process for key intermediate sulfonated saccharide of Gemcitabine
A compound, methylsulfonyl technology, applied in the field of drug synthesis, can solve the problems of low reaction yield, low selectivity, and low utilization rate of raw materials, and achieve high reaction efficiency, low isomer content, and high purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
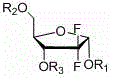
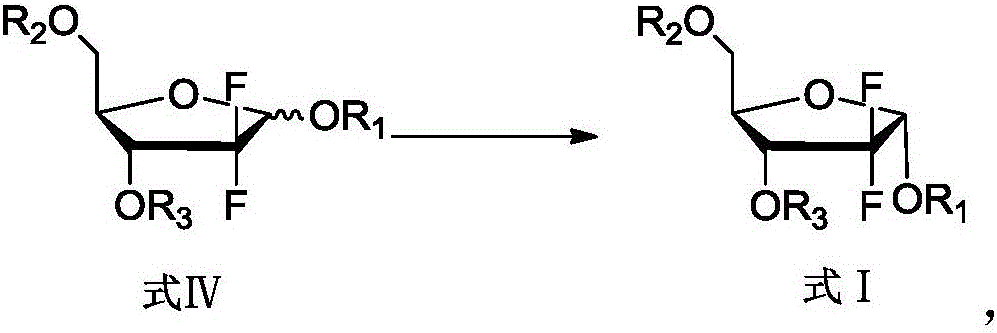
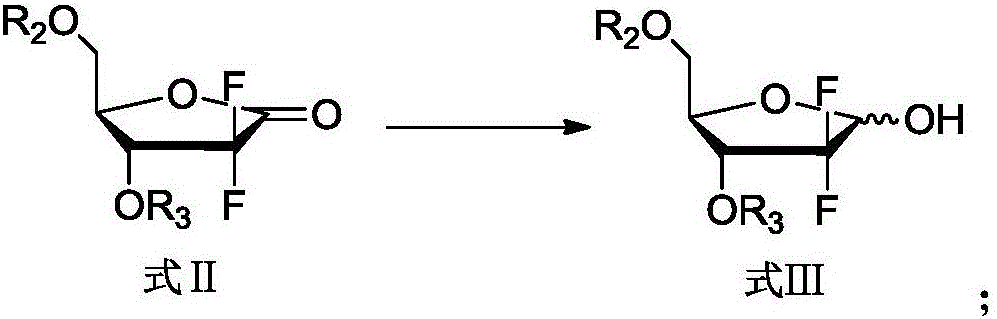
[0033] Add 45 mL of ethyl acetate and 15 mL of tetrahydrofuran into the reaction flask, add 10 g of 2-deoxy-2,2-difluoro-D-erythro-pentofuranose-1-one-3,5-dibenzoate, and anhydrous Zinc chloride 1.8, stir to dissolve, add 2 g of tert-butanol, add 0.67 g of sodium borohydride under temperature control below 15°C, and react for 1 to 1.5 hours. After the reaction is complete, add 40 mL of dilute hydrochloric acid, stir for 10 minutes, let stand to separate layers, discard the water layer, and wash the organic layer with 20 mL of saturated brine and 20 mL of saturated aqueous sodium bicarbonate solution, dry over anhydrous magnesium sulfate, filter, and concentrate to obtain 2- Deoxy-2,2-difluoro-D-ribofuranose-3,5-dibenzoate 10g, α / β=3.7, yield 100%.
Embodiment 2
[0035]
[0036] Add 45 mL of ethyl acetate and 15 mL of tetrahydrofuran into the reaction flask, add 10 g of 2-deoxy-2,2-difluoro-D-erythro-pentofuranose-1-one-3,5-diacetate, anhydrous chlorine Zinc chloride 1.8, stir to dissolve, add 2 g of tert-butanol, add 0.67 g of sodium borohydride under temperature control below 15°C, and react for 1 to 1.5 hours. After the reaction is complete, add 40 mL of dilute hydrochloric acid, stir for 10 minutes, let stand to separate layers, discard the water layer, and wash the organic layer with 20 mL of saturated brine and 20 mL of saturated aqueous sodium bicarbonate solution, dry over anhydrous magnesium sulfate, filter, and concentrate to obtain 2- Deoxy-2,2-difluoro-D-ribofuranose-3,5-diacetate 10g, α / β=3.1, yield 100%.
Embodiment 3
[0038]
[0039] Add 60 mL of ethyl acetate to the reaction flask, add 10 g of 2-deoxy-2,2-difluoro-D-erythro-pentofuranose-1-one-3,5-dibenzoate, anhydrous zinc chloride 1.8, stir to dissolve, add 2 g of tert-butanol, add 0.67 g of sodium borohydride under temperature control below 15°C, and react for 1 to 1.5 hours. After the reaction is complete, add 40 mL of dilute hydrochloric acid, stir for 10 minutes, let stand to separate layers, discard the water layer, and wash the organic layer with 20 mL of saturated brine and 20 mL of saturated aqueous sodium bicarbonate solution, dry over anhydrous magnesium sulfate, filter, and concentrate to obtain 2- Deoxy-2,2-difluoro-D-ribofuranose-3,5-dibenzoate 10g, α / β=3.1, yield 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com