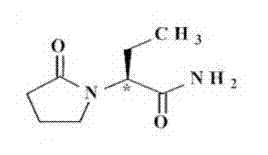
Levetiracetam injection
A technology for injection and water for injection, which is applied in the directions of non-active ingredients medical preparations, drug delivery, organic active ingredients, etc., can solve the problems of clinical hidden dangers and high levetiracetam acid content, and achieves reduction of hidden dangers and considerable economy. Benefits and social benefits, the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] prescription:
[0021] Raw material name 1000 doses (g) Proportion of raw and auxiliary materials (%) Levetiracetam 500g 10.0% cysteine 250g 5.0% Sodium chloride 10g 0.2% Sodium acetate 45g 0.9% acetic acid 15g 0.3% Water for Injection 4180g 83.6%
[0022] Preparation:
[0023] Take 70% of the water for injection and put it in a stainless steel bucket, weigh levetiracetam, cysteine, sodium acetate, and sodium chloride according to the prescribed amount and add them to the water for injection, stir for 30 minutes to dissolve and mix well, add acetic acid to adjust the pH When the value reaches 6.5, add 0.02% activated carbon for injection, keep the water temperature at 70°C, stir for 15 minutes, filter and decarbonize while hot, let it cool to room temperature, measure the solution content and pH value, add water for injection to the full amount according to the measurement results, mix well, Filter through a 0....
Embodiment 2
[0025] prescription:
[0026] Raw material name 1000 doses (g) Proportion of raw and auxiliary materials (%) Levetiracetam 500g 10.0% cysteine 300g 6.0% Sodium chloride 5g 0.1% Sodium acetate 60g 1.2% acetic acid 10g 0.2% Water for Injection 4125g 82.5%
[0027] Preparation:
[0028] Take 70% of the water for injection and put it in a stainless steel bucket, weigh levetiracetam, cysteine, sodium acetate, and sodium chloride according to the prescribed amount and add them to the water for injection, stir for 30 minutes to dissolve and mix well, add acetic acid to adjust the pH When the value reaches 6.0, add 0.02% activated carbon for injection, keep the water temperature at 70 °C, stir for 15 minutes, filter and decarbonize while hot, let it cool to room temperature, measure the solution content and pH value, add water for injection to the full amount according to the measurement results, mix well, Filter through a 0....
Embodiment 3
[0030] prescription:
[0031] Raw material name 1000 doses (g) Proportion of raw and auxiliary materials (%) Levetiracetam 500g 10.0% cysteine 400g 8.0% Sodium chloride 5g 0.1% Sodium acetate 60g 1.2% acetic acid 5g 0.1% Water for Injection 4030g 80.6%
[0032] Preparation:
[0033]Take 70% of the water for injection and put it in a stainless steel bucket, weigh levetiracetam, cysteine, sodium acetate, and sodium chloride according to the prescribed amount and add them to the water for injection, stir for 30 minutes to dissolve and mix well, add acetic acid to adjust the pH When the value reaches 5.5, add 0.02% activated carbon for injection, keep the water temperature at 70 °C, stir for 15 minutes, filter and decarbonize while hot, let it cool to room temperature, measure the solution content and pH value, add water for injection to the full amount according to the measurement results, mix well, Filter through a 0.22...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com