Levetiracetam sustained-release tablets and preparation method thereof
A technology of sustained-release tablets and sustained-release materials, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations of inactive ingredients, etc., can solve the problem of the reduced overall application effect of levetiracetam sustained-release tablets, and the release porosity. In order to achieve the effect of maintaining sustained release effect, simple operation and easy control, and stable content of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
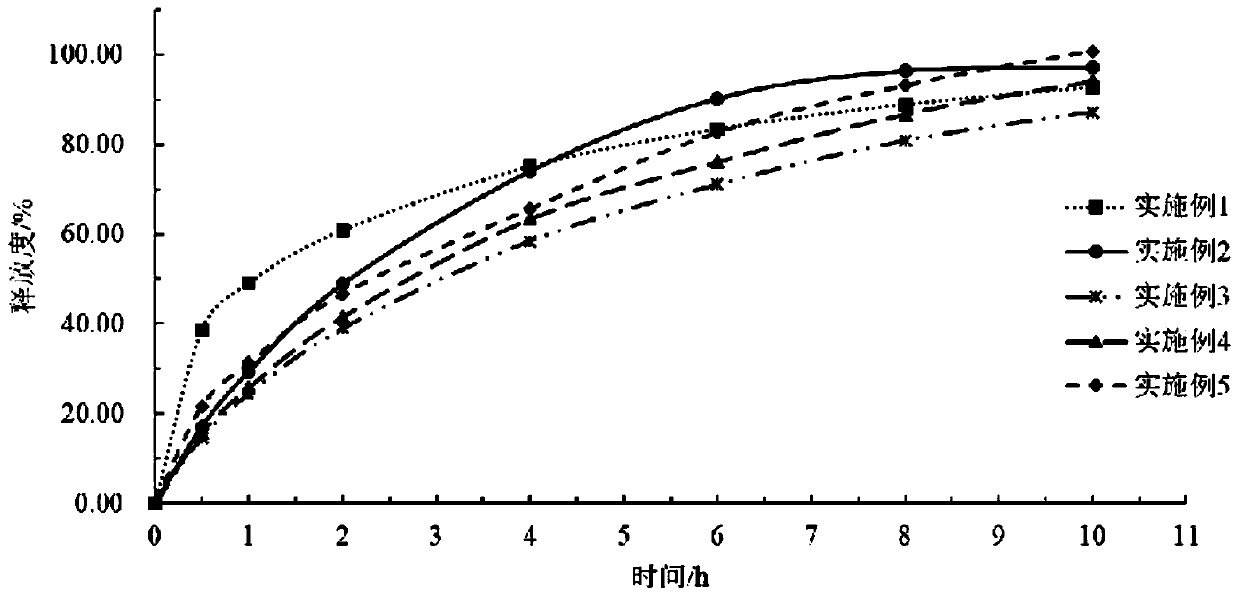
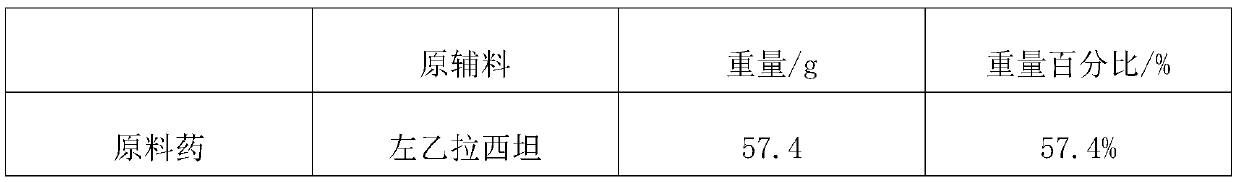
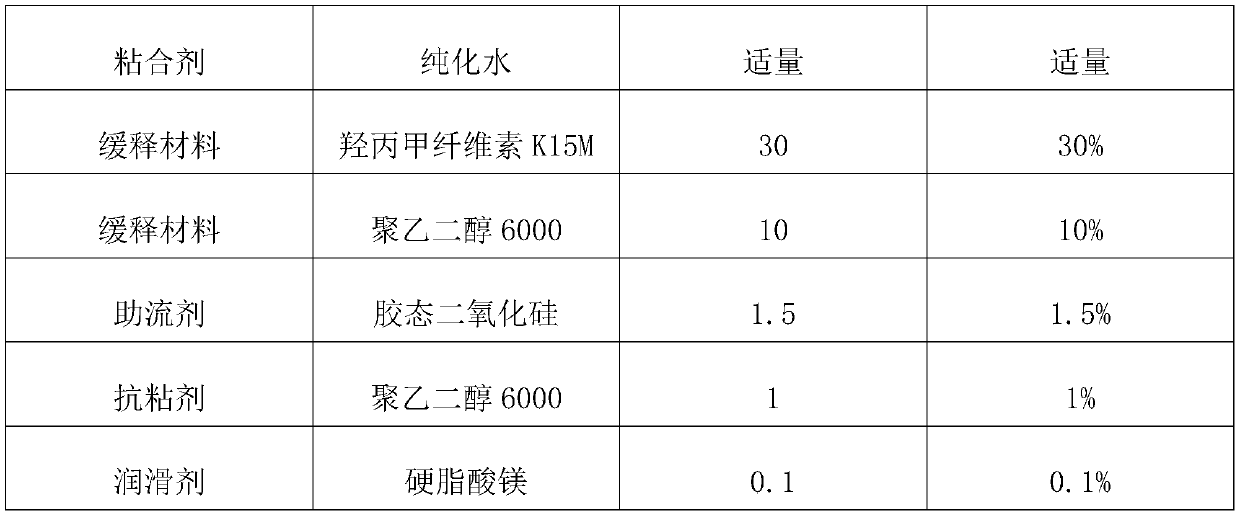
[0050] Embodiment 1: a kind of levetiracetam slow-release tablet, each component and corresponding parts by weight thereof are as shown in Table 1, and the preparation process of levetiracetam slow-release tablet (specification: 750mg) is as follows :
[0051] (1) Pretreatment: use a pulverizer to pulverize each material, and control the particle size of each material at 100-180 μm;
[0052] (2) Preparation of binder solution: Take 0.5 g of hypromellose E5, add 30 mL of boiling water, stir vigorously, add the remaining amount of hypromellose, make up 100 g with cold water, and stir evenly to obtain a mass concentration of 1%. Hypromellose aqueous solution;
[0053] (3) Granulation: according to the ratio, take all the raw materials and slow-release materials and mix them in a wet granulator. The mixing time is 10 minutes. Add the binder solution at a uniform speed and granulate with a 24 mesh sieve. Drying, the moisture content of the granules is controlled below 2%; then us...
Embodiment 2
[0058] Embodiment 2: a kind of levetiracetam slow-release tablet, each component and its corresponding parts by weight are as shown in Table 2, the preparation process of levetiracetam slow-release tablet (specification: 750mg) is as follows :
[0059] (1) Pretreatment: use a pulverizer to pulverize each material, and control the particle size of each material at 100-180 μm;
[0060] (2) Granulation: according to the ratio, take the raw material drug and hypromellose K15M with a ratio of 50% and place it in a fluidized bed granulation coating dryer for mixing. , until the powder is in a fluidized state, dry at 40°C, and control the moisture content of the granules below 2%; then use a 32-mesh sieve to sieve the granules;
[0061] (3) Mixing: Take the granules in proportion and mix them with the remaining slow-release materials, glidants, anti-sticking agents and lubricants in a mixer, and the mixing time is 20 minutes;
[0062] (4) Tablet compression: The tablet weight was c...
Embodiment 3
[0065] Embodiment 3: a kind of levetiracetam slow-release tablet, each component and its corresponding parts by weight are as shown in Table 3, the preparation process of levetiracetam slow-release tablet (specification: 750mg) is as follows :
[0066] (1) Pretreatment: use a pulverizer to pulverize each material, and control the particle size of each material at 100-180 μm;
[0067] (2) Granulation: Take the raw material according to the proportion and place it in a fluidized bed granulation coating dryer, spray water into it at a constant speed through a peristaltic pump until the powder is in a fluidized state, dry at 40°C, and control the moisture content of the granules at 2%. Below; then granulate with 32 mesh sieve;
[0068] (3) Mixing: Take the granules in proportion and mix them with slow-release materials, glidants, anti-sticking agents and lubricants in a mixer, and the mixing time is 20 minutes;
[0069] (4) Tablet compression: The tablet weight was controlled ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com