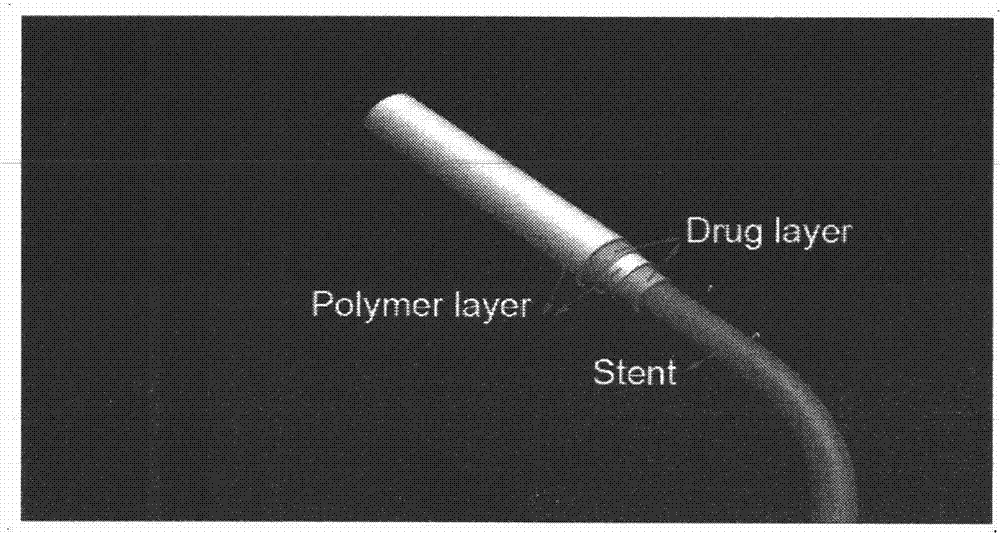
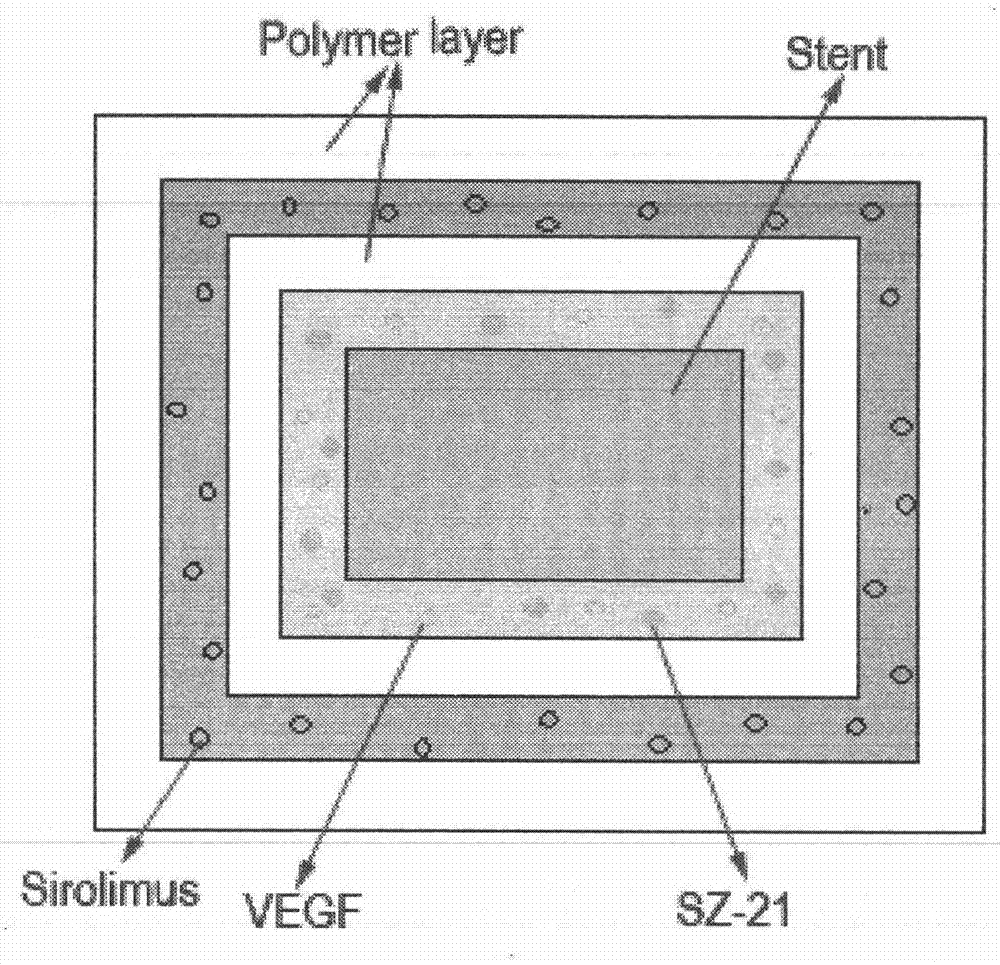
Preparation method of multi-coating drug eluting intravascular stent
A vascular stent and multi-layer coating technology, which is applied in the field of medical devices, can solve the problems of high thrombus formation rate, and achieve the effects of high production efficiency, good biocompatibility, and good sustained-release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In this embodiment, the first layer of paint is a chitosan acetate solution of the platelet membrane glycoprotein receptor IIIa antagonist sz-21, and the third layer of paint is a solution of rapamycin and PLLA, which are sprayed under corresponding experimental conditions. The specific operation process is as follows:
[0025] (1) Put the 316L stainless steel bare metal bracket into 75% ethanol solution, acetone, and distilled water for 5-10 minutes, and then put it into 75% ethanol solution, acetone, and distilled water for 5-10 minutes to remove the residual oil, dust and other impurities on the surface of the bracket, and then soak it in chromic acid lotion 10-20min, then thoroughly cleaned with distilled water for 20min by ultrasonic vibration, and then dried in a vacuum oven at 50°C for 24 hours.
[0026] (2) Take by weighing 5mg sz-21 powder and be dissolved in 5ml1% acetic acid solution, mix and make medicine solution, then take by weighing 5mg chitosan and diss...
Embodiment 2
[0034] The first coat of present embodiment is the chitosan acetic acid solution of pro-vascular growth factor VEGF, platelet membrane glycoprotein receptor IIIa antagonist sz-21, and the third coat is rapamycin and PLLA solution, under corresponding experimental condition down to spray. The specific operation process is as follows:
[0035] (1) Put the 316L stainless steel bare metal bracket into 75% ethanol solution, acetone, and distilled water for 5-10 minutes, and then put it into 75% ethanol solution, acetone, and distilled water for 5-10 minutes to remove the residual oil, dust and other impurities on the surface of the bracket, and then soak it in chromic acid lotion 10-20min, then thoroughly cleaned with distilled water for 20min by ultrasonic vibration, and then dried in a vacuum oven at 50°C for 24 hours.
[0036](2) Dissolve 1mg VEGF and 5mg sz-21 powder in 5ml 1% acetic acid solution, mix well to make a drug solution, then weigh 5mg chitosan and dissolve it in th...
Embodiment 3
[0044] The first coat of present embodiment is the chitosan acetic acid solution of pro-vascular growth factor VEGF, platelet membrane glycoprotein receptor IIIa antagonist sz-21, and the third coat is docetaxel and PLLA solution, under corresponding experimental conditions down to spray. The specific operation process is as follows:
[0045] (1) Put the 316L stainless steel bare metal bracket into 75% ethanol solution, acetone, and distilled water for 5-10 minutes, and then put it into 75% ethanol solution, acetone, and distilled water for 5-10 minutes to remove the residual oil, dust and other impurities on the surface of the bracket, and then soak it in chromic acid lotion 10-20min, then thoroughly cleaned with distilled water for 20min by ultrasonic vibration, and then dried in a vacuum oven at 50°C for 24 hours.
[0046] (2) Dissolve 1mg VEGF and 5mg sz-21 powder in 5ml 1% acetic acid solution, mix well to make a drug solution, then weigh 5mg chitosan and dissolve it in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com