Method for detecting levetiracetam enantiomer in levetiracetam raw material or sodium chloride injection
A technology of sodium chloride injection and enantiomers, which is applied in the field of detection of levetiracetam enantiomers, and can solve the problem of large polarity differences and symmetrical peak shapes of levetiracetam isomers Poor performance, undetectable and other problems, to achieve the effect of high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
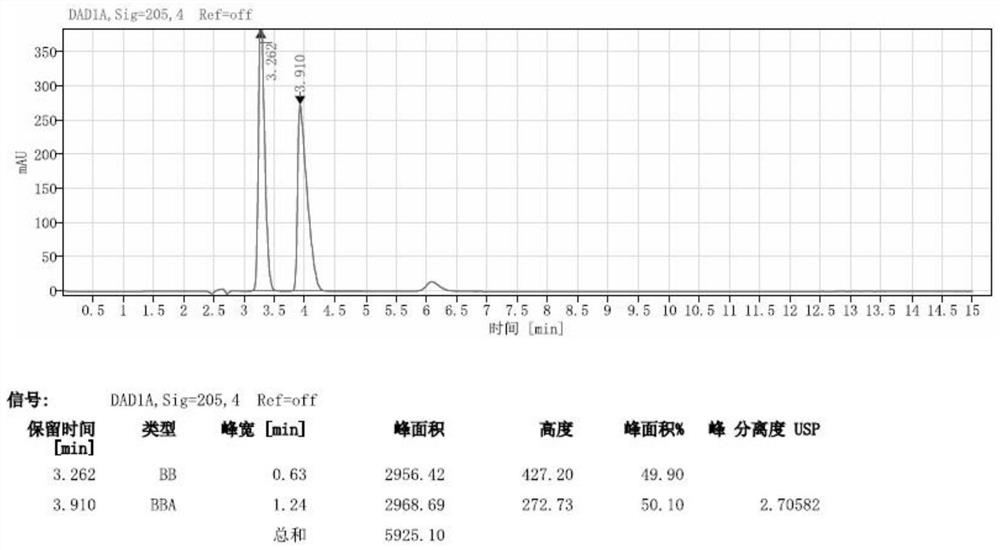
[0057] Embodiment 1: System suitability experiment
[0058] Instrument: Agilent1260 high performance liquid chromatography; Chromatographic column: CHIRALPAK AGP (150mm×4mm, 5μm).
[0059] Chemical reagents: ammonia water (AR, Nanjing Chemical Laboratory); potassium dihydrogen phosphate (AR, Nanjing Chemical Laboratory); water (ultrapure water, self-made).
[0060] Sample to be tested: levetiracetam sodium chloride injection (manufacturer: Yangzijiang Pharmaceutical Group Nanjing Hailing Pharmaceutical Co., Ltd., batch number 18032221).
[0061] Reference substance: levetiracetam (content: 100%, manufacturer: European Pharmacopoeia, batch number: 3.0); levetiracetam enantiomer (content: 100%, manufacturer: European Pharmacopoeia, batch number: 3.0); Levetiracetam racemate (content: 100%, manufacturer: European Pharmacopoeia, batch number: R028T0).
[0062] System suitability solution: Dissolve the levetiracetam racemate reference substance in water to make a system suitabili...
Embodiment 2
[0073] Embodiment 2: specificity experiment
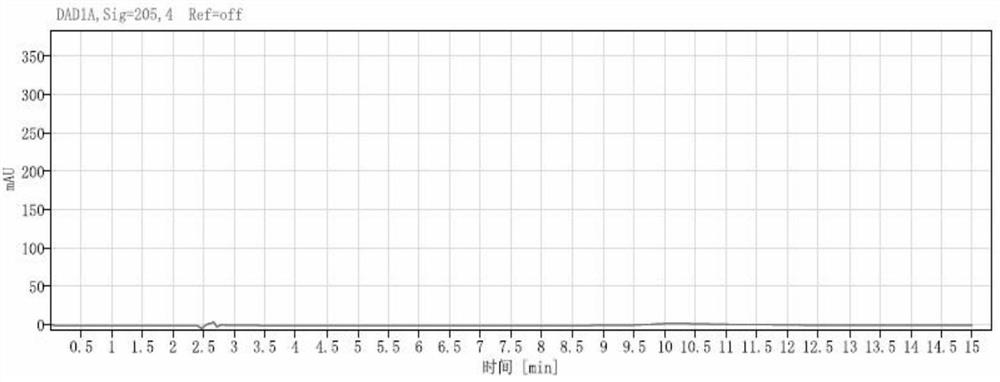
[0074] (1) Selectivity and interference test
[0075] Blank solution: water.
[0076] Blank auxiliary material solution: Take 1 mL of blank auxiliary material (take 8.2 g of sodium chloride, 1.64 g of sodium acetate, add water to dissolve and dilute to 1000 mL, adjust the pH to 5.5 with glacial acetic acid), put it in a 10 mL measuring bottle, add water to dilute to the mark, shake well, Instantly.
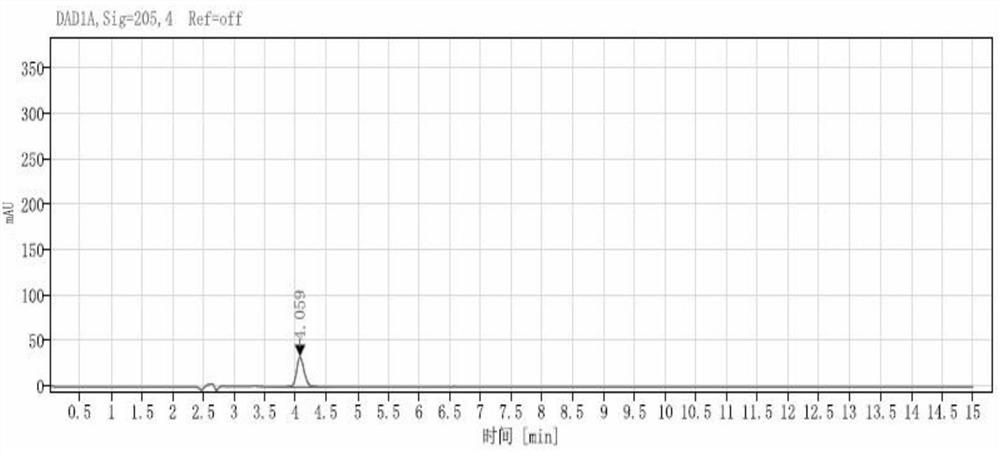
[0077] System suitability solution: Weigh the levetiracetam racemate reference substance and dissolve it in water to form a system suitability solution, wherein the concentrations of levetiracetam and levetiracetam enantiomers are both 0.05 mg / mL.
[0078] Levetiracetam enantiomer reference solution: Take about 4 mg of levetiracetam R isomer reference substance, weigh it accurately, add water to dissolve and dilute to 100 mL, shake well, and use as levetiracetam R Isomer reference stock solution. Precisely measure 1 mL of levetira...
Embodiment 3
[0090] Embodiment 3: Stability experiment
[0091] Prepare need testing solution and levetiracetam enantiomer reference substance solution according to embodiment 2, then need testing solution and levetiracetam enantiomer reference substance solution at room temperature, Place 48h, during this placement, need testing solution and levetiracetam enantiomer reference substance solution are carried out high-performance liquid chromatography detection respectively, detection condition is the same as embodiment 1, and detection result is as shown in table 3 :
[0092] Table 3 Stability Test
[0093]
[0094] It can be seen from Table 3 that the test solution and the levetiracetam enantiomer reference solution are still stable at room temperature for 48 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com