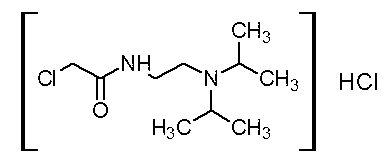
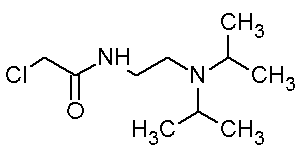
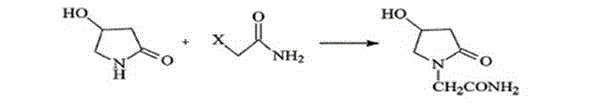
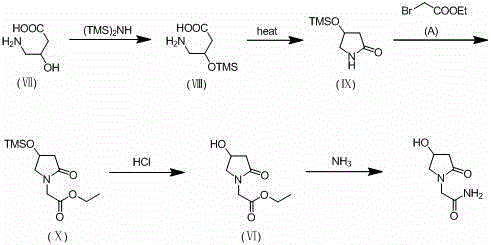
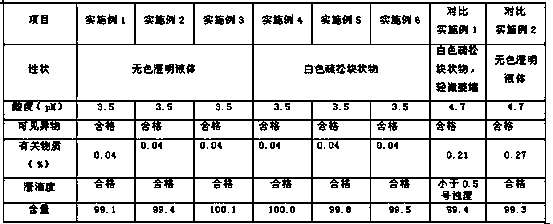
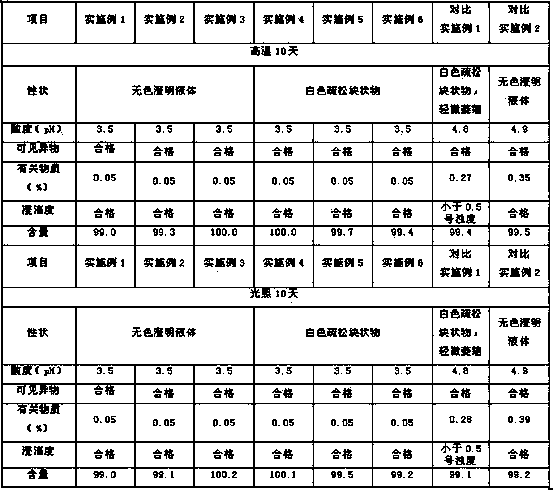
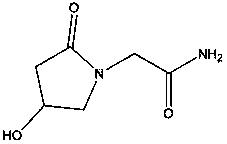
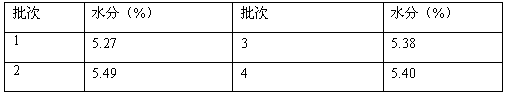
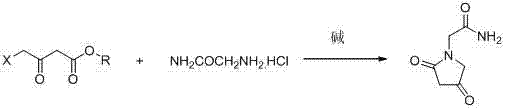Patents
Literature
85 results about "Racetam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Racetams are a class of drugs that share a pyrrolidone nucleus. Some, such as piracetam, are considered nootropics. Some such as aniracetam, oxiracetam, pramiracetam and phenylpiracetam are also nootropics. Others such as levetiracetam and seletracetam are anticonvulsants.
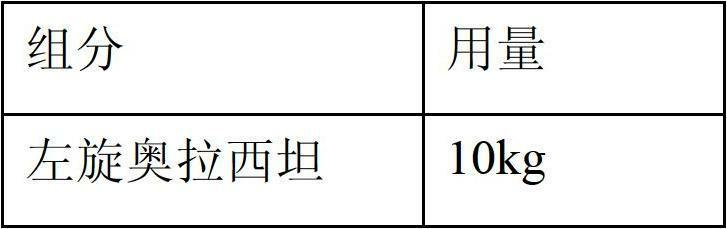
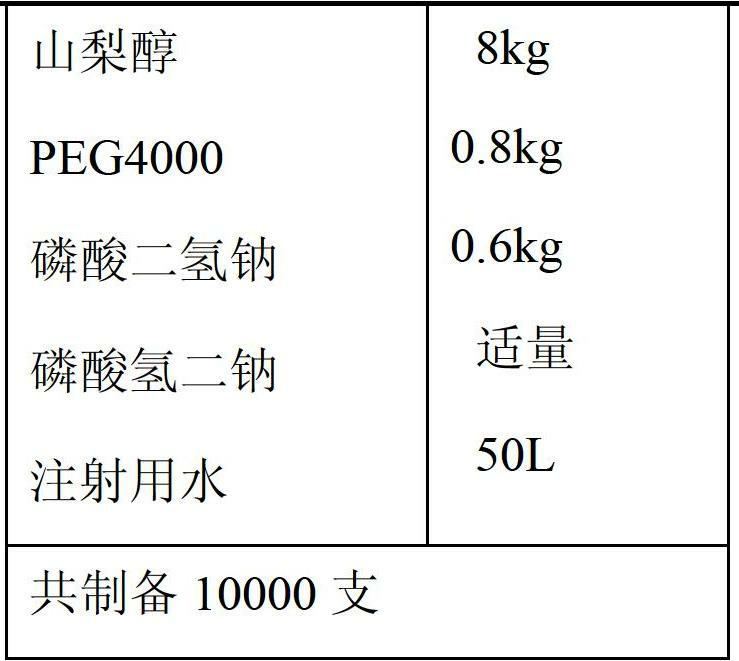
Application of levo-oxiracetam in preparation of medicine for treating memory and intelligence disturbance
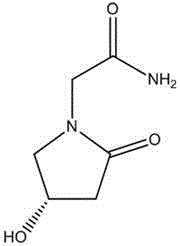
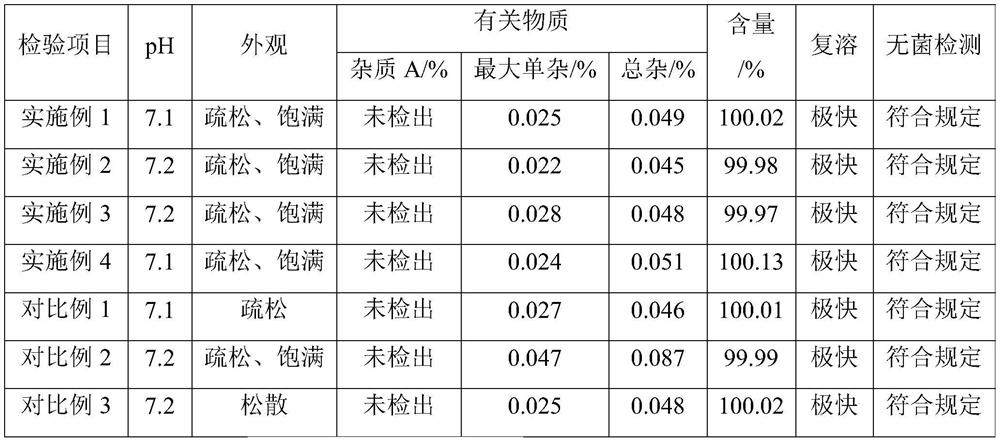
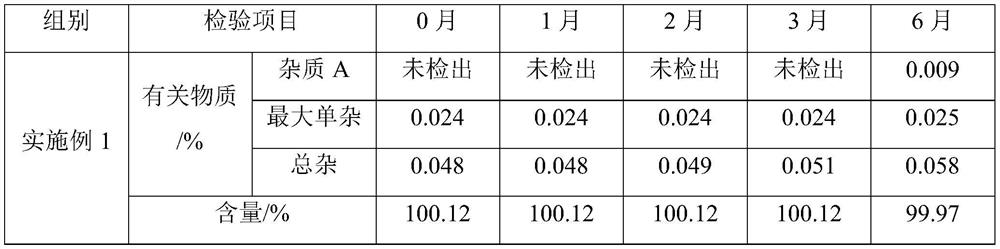
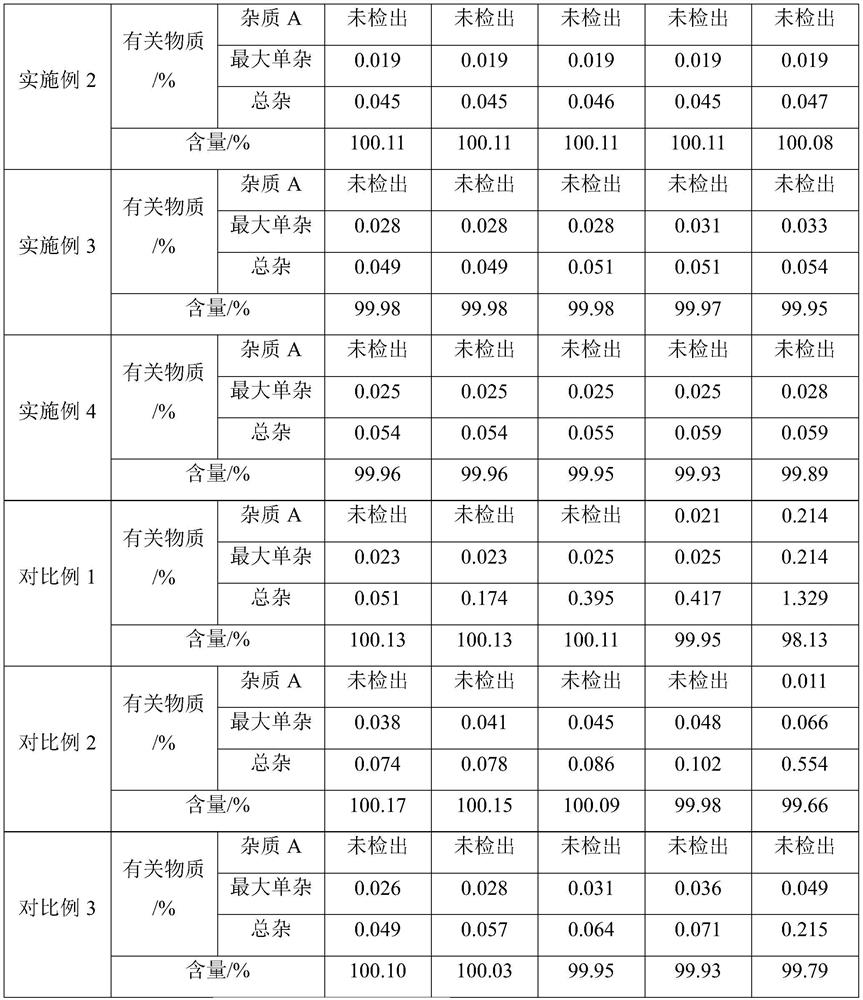
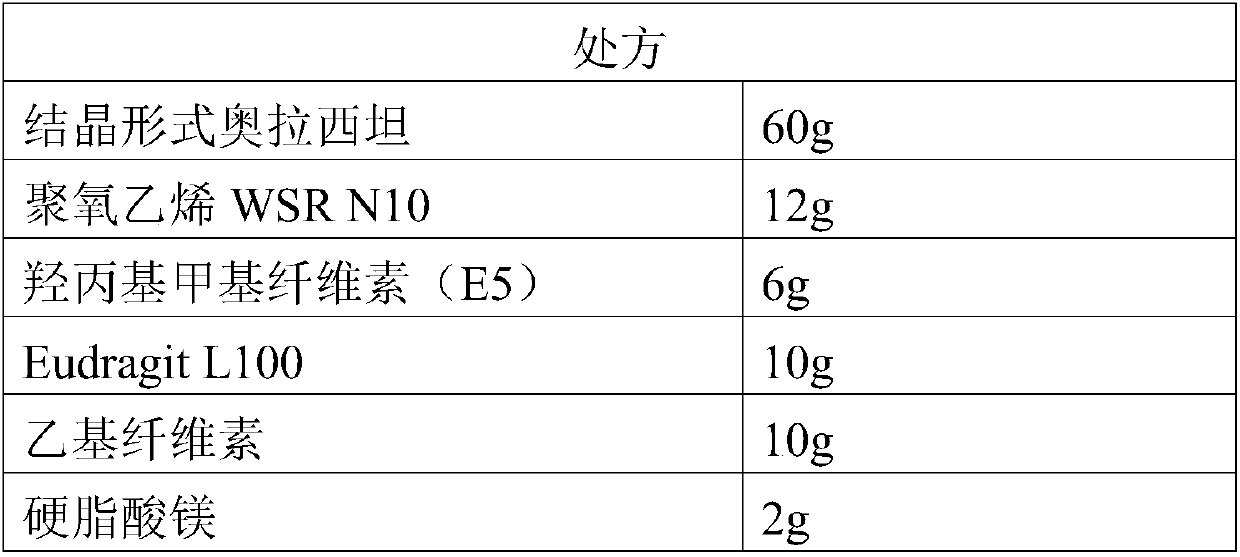
The invention relates to new use of levo-oxiracetam in pharmaceutical field, and in particular relates to application of levo-oxiracetam in preparation of a medicine for treating memory and intelligence disturbance. The experiment result shows that the levo-oxiracetam is a main active ingredient for playing efficacy in oxiracetam, the clinical dosage can be greatly reduced by singly using the levo-oxiracetam, and the potential toxic and side effect is reduced. According to the invention, the levo-oxiracetam is a single active ingredient, the raw material purity is greater than 99.5% so as to effectively avoid the toxicity risk caused by other impurities in the medicine, the pharmacy is safer, the medicine quality is more controllable, and the curative effect is more precise.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +2
Freeze-dried powder injection of L-oxiracetam and process for preparing freeze-dried powder injection
ActiveCN102670527AFast sublimationShorten freeze-drying timePowder deliveryOrganic active ingredientsPolyethylene glycolEngineering
The invention relates to a freeze-dried powder injection of L-oxiracetam and a process for preparing the freeze-dried powder injection. The freeze-dried powder injection comprises, by weight, 1 part of the L-oxiracetam, 0.1 to 10 parts of an excipient, 0.01 to 1 part of an antisticking agent and 0.01 to 0.5 part of a pH regulator, wherein polyethylene glycol (PEG) series of products serve as the antisticking agent preferably. The freeze-dried powder injection which is prepared on the basis of a prescription according to the process has the advantages of being short in freeze-drying time, attractive in appearance, short in redissolving time and high in stability.
Owner:南京博德生物制药有限公司
Preparing method of oxiracetam injection and products thereof
InactiveCN1424034AReduce workloadThe preparation process is feasibleOrganic active ingredientsDrug compositionsActivated carbonMedical prescription
An oxiracetam injection is prepared from oxiracetam, gluclose (or sodium chloride) for injection and water for injection through proportioning, dissolving the glucose or sodium chloride for injection in the water for injection, adding activated carbon, heating, holding the temp. filtering, adding oxiracetam to the filtrate, stirring for dissolving, adding the water for injection, regulating pH value, fine filtering, bottling and sterilizing at 105-126.5 deg.C.
Owner:诸葛华明 +1
Oxiracetam compound with steady crystal form
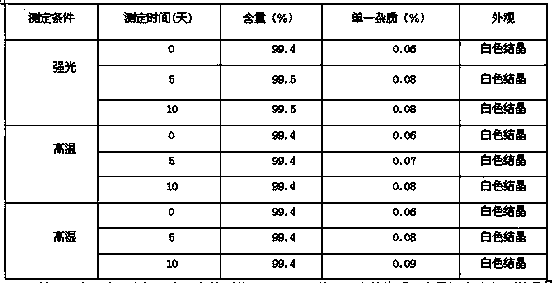
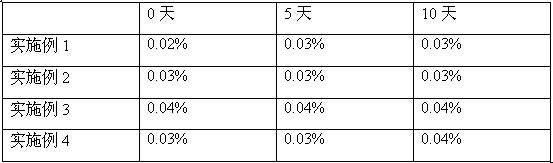
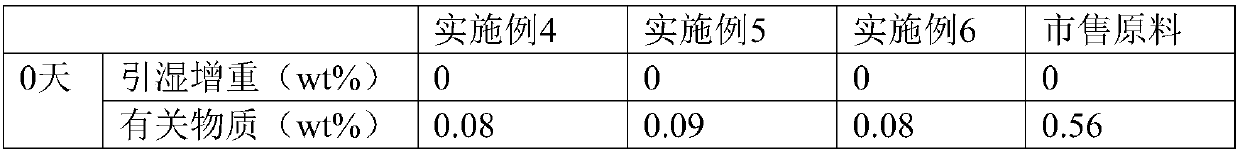
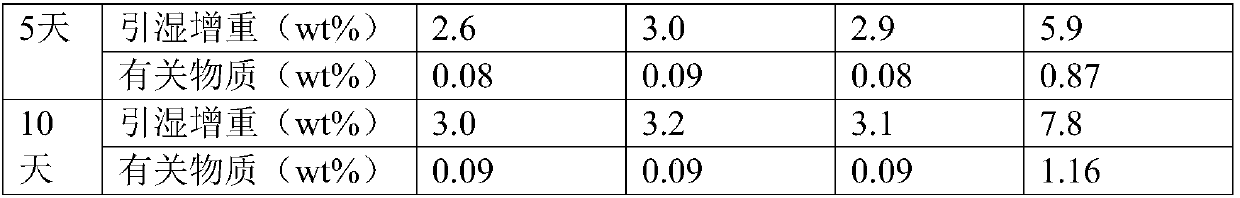
The invention belongs to the technical field of medicine, and particularly relates to an oxiracetam compound. The oxiracetam compound provided by the invention contains semi-crystalline water. The oxiracetam compound has the advantages that related substances are small, the purity is high, the stability is good, and a moisture-absorption and weight-gaining effect is not obvious even if under a high humidity condition.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Oxiracetam compound and new method thereof
The invention relates to an oxiracetam compound and a new method thereof. In the method, 3-chloro-2-hydroxy propionitrile is used as an initial raw material. The method comprises the following steps of: synthesizing 4-hydroxyl-2-pyrrolidone through 4-chloro-3-hydroxyl-butylamide; and then synthesizing oxiracetam. The invention overcomes the defects of complicated preparation process, trouble steps, high cost, low product purity and difficult purification of the prior art.
Owner:HAINAN LINGKANG PHARMA CO LTD
Oxiracetam drug activity composition and preparation method thereof
ActiveCN102846600AComply with medicinal requirementsQuality improvementOrganic active ingredientsNervous disorderClinical efficacyEthyl acetate
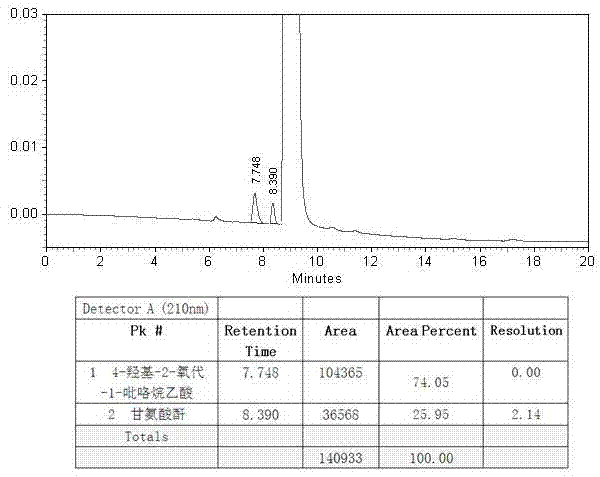
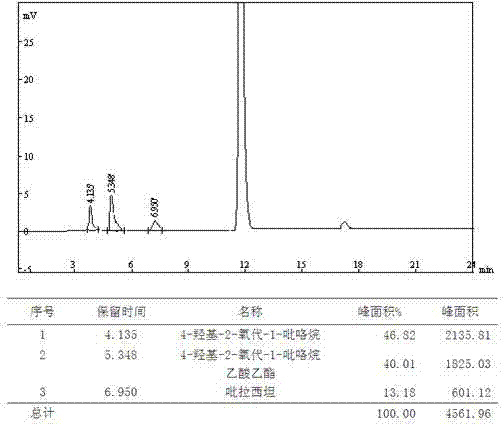
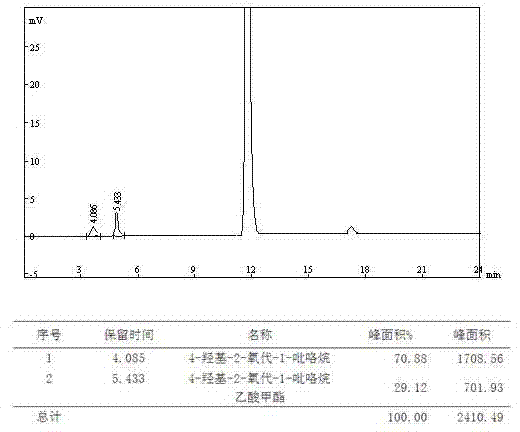
The present invention provides an oxiracetam drug activity composition, which comprises the following components: a component I, a component II and a component III, wherein the component I is oxiracetam, the component I content is more than or equal to 98.0%, the component II is glycine anhydride, the component II content is more than 0 and is less than or equal to 0.3%, the component III is one or a plurality materials selected from 4-hydroxy-2-oxo-1-pyrrolidineacetic acid, 4-hydroxy-2-oxo-1-pyrrolidine, ethyl 4-hydroxy-2-oxopyrrolidine-1-acetate, methyl 4-hydroxy-2-oxopyrrolidine-1-acetate, and piracetam, the component III content is more than 0 and is less than or equal to 1.5%, the 4-hydroxy-2-oxo-1-pyrrolidineacetic acid content is more than 0 and is less than or equal to 0.5%, and the total content of the component II and the component III is less than or equal to 1.5%. The drug activity composition of the present invention has stable quality, and can completely meet quality requirements on the drug activity composition by oxiracetam preparations. In addition, the prepared preparation has characteristics of safety, effectiveness, and controllable quality, and clinical therapy effects and medication safety of the oxiracetam preparation are ensured.
Owner:CSPC OUYI PHARM CO LTD
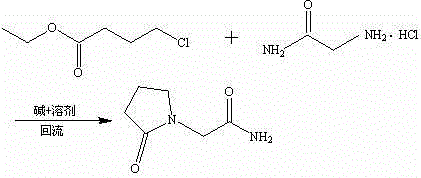
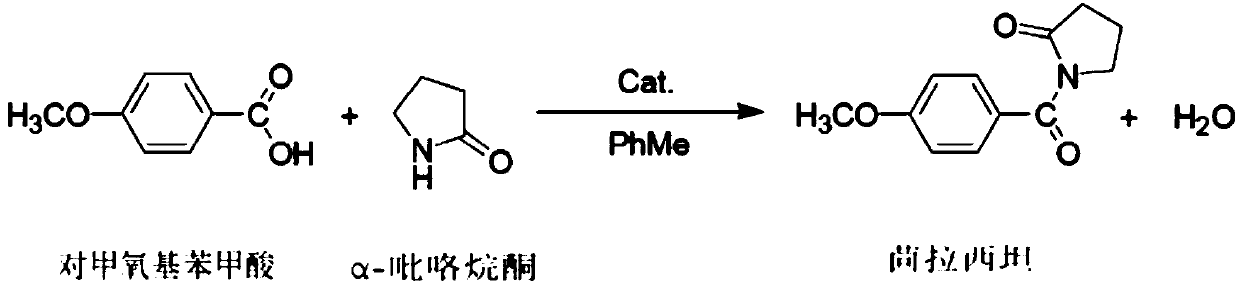
Novel piracetam synthetic method
InactiveCN102718691AThe reaction steps are simpleMild reaction conditionsOrganic chemistryChemical synthesisOrganosolv
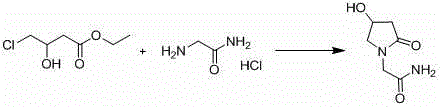
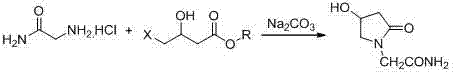
The invention belongs to the field of chemical synthetic techniques, in particular relates to a novel piracetam synthetic method. The method comprises the following steps of: mixing ethyl-4-chloro-n-butanoate, glycinamide hydrochloride, alkaline and an alcohol organic solvent, and heating and reflowing for 20 to 30 hours, and thermally filtering, evaporating to remove the solvent under the reduced pressure, and then recrystallizing, filtering and drying the residues to obtain white or almost white crystal which is piracetam, wherein the mole ratio of ethyl-4-chloro-n-butanoate to glycinamide hydrochloride to alkaline is 1: (1.1-1.8): (1.0-2.0). The method is simple and short in reaction step, and mild in reaction condition, is convenient to operate for production; and moreover, the method is environment-friendly, and the synthetic raw materials and solvents are cheap and easy to obtain, thus the method is suitable for industrial production.
Owner:SHANGHAI MODERN HASEN SHANGQIU PHARMA
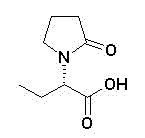
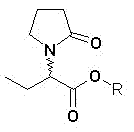
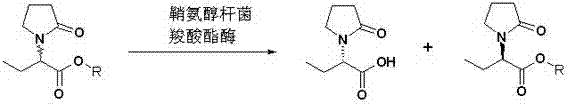
Sphingobacterium and method for preparing levetiracetam acid by utilizing same
InactiveCN102851238AMild reaction conditionsEasy to trainBacteriaMicroorganism based processesSphingobium chlorophenolicumHydrolysis
The invention discloses Sphingobacterium (sp.) SIT102 with the preservation number of CGMCC NO.6158 and a method for performing enantioselective hydrolysis to generate levetiracetam acid by utilizing the Sphingobacterium SIT102 serving as biocatalyst to catalyze racemization etiracetam acid esters. The method for preparing the levetiracetam acid comprises the steps of placing Sphingobacterium SIT102 cells into buffered solution, adding the racemization etiracetam acid esters into the buffered solution, performing the enantioselective hydrolysis through catalysis to obtain the levetiracetam acid. According to the method for preparing the levetiracetam acid by utilizing the Sphingobacterium SIT102 serving as the biocatalyst, the used biocatalyst is easy to prepare, the reaction condition is moderate, the yield of the levetiracetam acid can reach 48%, the enantiomer excess reaches 96%, and the production cost is low. Therefore, the method for preparing the levetiracetam acid by utilizing the Sphingobacterium has considerable industrial application development prospects.
Owner:SHANGHAI INST OF TECH
Method for large-scale preparation of pramiracetam
InactiveCN103012236ASynthetic process yield is highHigh purityOrganic chemistryProcess engineeringIndustrial engineering
The invention relates to a method for large-scale preparation of pramiracetam. The method comprises the following steps of: carrying out a condensation reaction, dissolving and filtering, re-dissolving and extracting, refluxing, freezing and suction-filtering. The method provided by the invention has simple process operation, is safe and pollution-free. Moreover, the prepared pramiracetam has high yield, high purity, easily obtained materials, and low production cost, and is suitable for large-scale production of the enterprises.
Owner:KAMP PHARMA
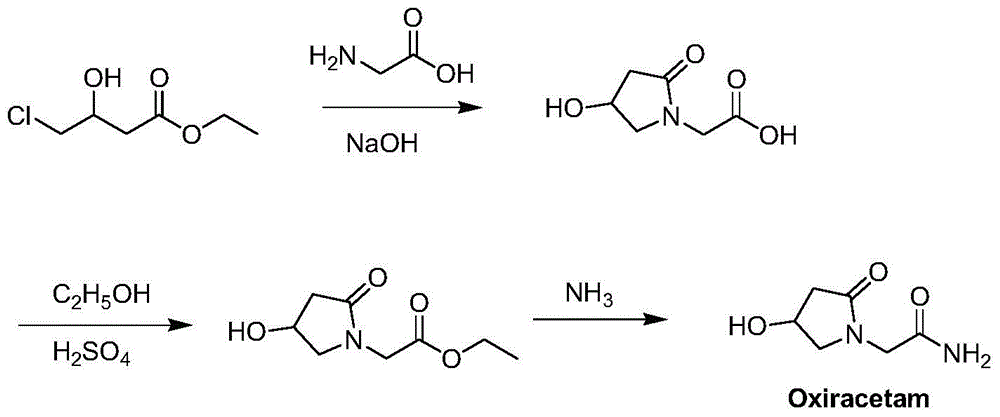
Synthesis method of oxiracetam
ActiveCN102746207AAvoid it happening againHigh purityOrganic chemistryBulk chemical productionBiochemical engineeringPyrrolidine
The invention provides a method for preparing oxiracetam, which comprises the steps of: reacting 2-(4-tertiary butyl disilyloxy-2-oxo pyrrolidine-1-yl) ethyl acetate or 2-(4-tertiary butyl diphenylsilyl-2-oxo pyrrolidine-1-yl) ethyl acetate as raw materials with low-molecular-weight halogenated acetic acid ester in a non-protonated solvent under basic condition, and respectively removing protecting groups to prepare oxiracetam. By adopting the method, the problems of the prior art that reaction conditions are rigor, byproducts are many, and the product purity and yield are low in the oxiracetam synthesis process are solved. The method is simple to operate, low in equipment requirements, and high in product purity and yield, and is particularly suitable for industrial production.
Owner:BRIGHTGENE PHARMA
Piracetam tablet composition with high drug loading capacity and preparation method thereof
ActiveCN111297818ASmall sizeLow friabilityOrganic active ingredientsNervous disorderSoluble FilmCombinatorial chemistry
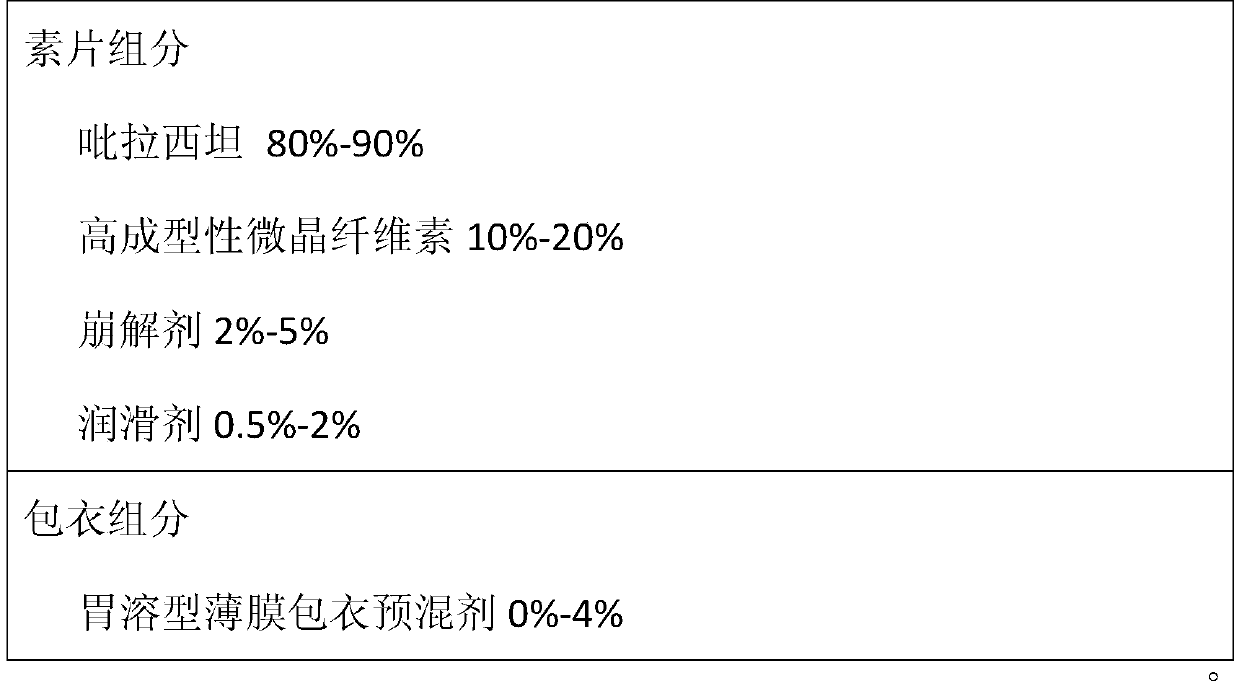
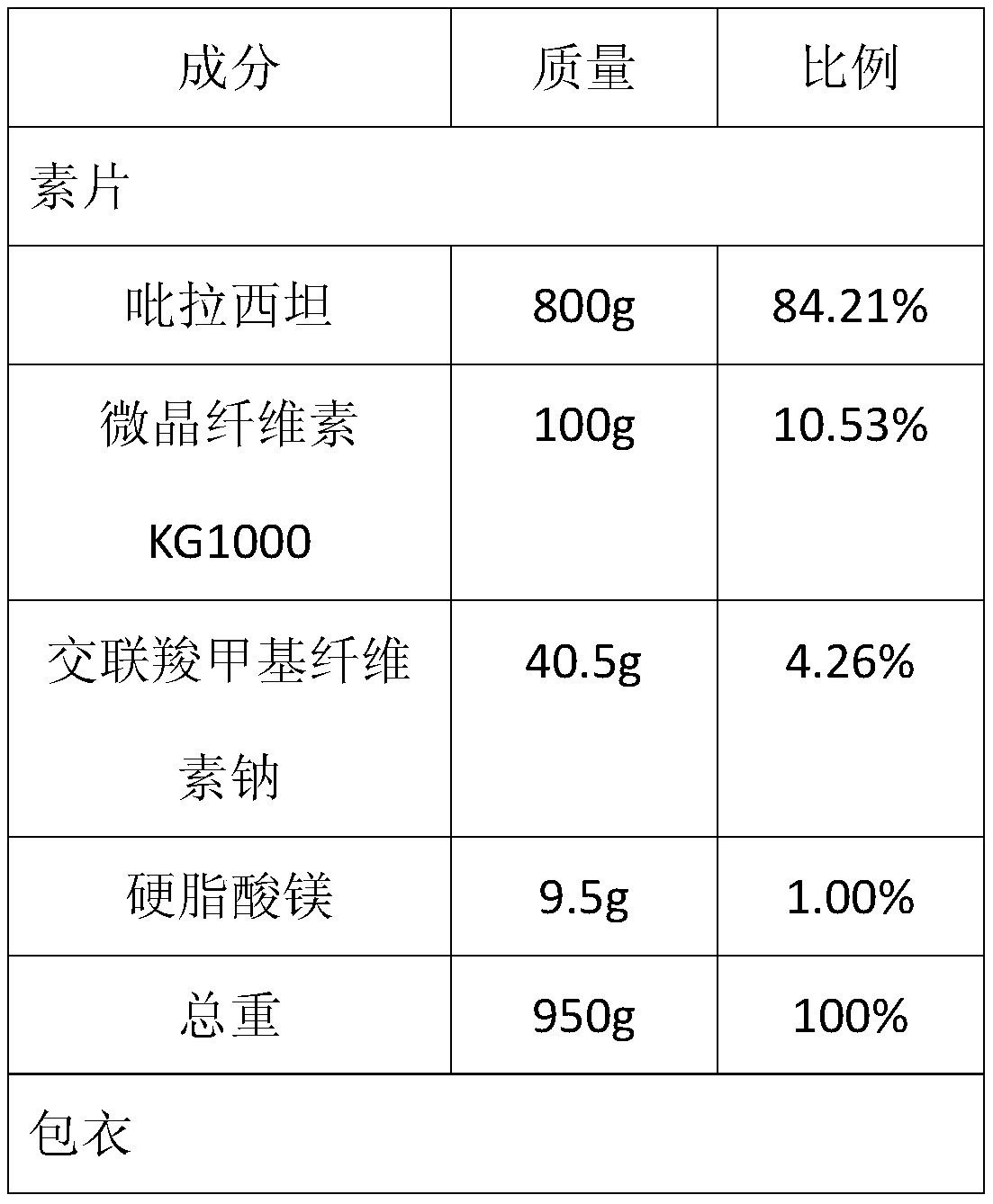
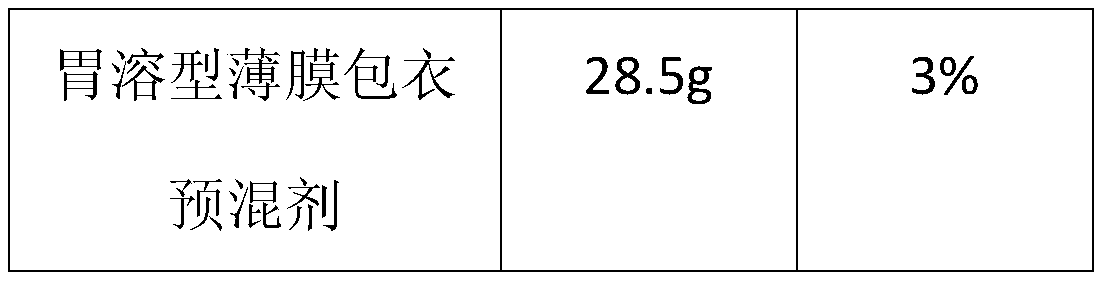
The invention provides a piracetam tablet composition with high drug loading capacity and a preparation method thereof. The piracetam tablet composition comprises 80-90% of piracetam, 10-20% of high-formability microcrystalline cellulose (CEOLUSTM), 2-5% of a disintegrating agent, 0.5-2% of a lubricant and 0-4% of a gastric-soluble film coating premix. The composition can effectively improve the drug loading capacity of a piracetam tablet and improve the problem of poor friability of a product due to improvement of the drug loading capacity. In addition, the invention also provides a preparation method for the piracetam tablet with the high drug loading capacity. The method provided by the invention is simple and convenient in process operation, high in production efficiency and applicableto industrial production.
Owner:CHANGZHOU PHARMA FACTORY
Oxiracetam injection composition and preparation method thereof
The invention relates to an oxiracetam injection composition and a preparation method thereof. The oxiracetam injection provided by the invention comprises 0.1-0.3 g of oxiracetam, 0.2-0.4 mg of sodium dihydrogen phosphate, 0.02-0.04 mg of sodium calcium edetate, 0.02-0.04 mg of glycine, 0.02-0.04 mg of vitamin B6, a pH value regulator (for regulating the pH value to the range from 4.5 to 5.0) and water for injection per 1 ml. The oxiracetam injection provided by the invention has the advantages of good quality and high stability.
Owner:HEBEI RENHE YIKANG PHARMA
Preparation method of aniracetam
InactiveCN107840816ANo pollution in the processSimple processOrganic chemistryCombinatorial chemistryAniracetam
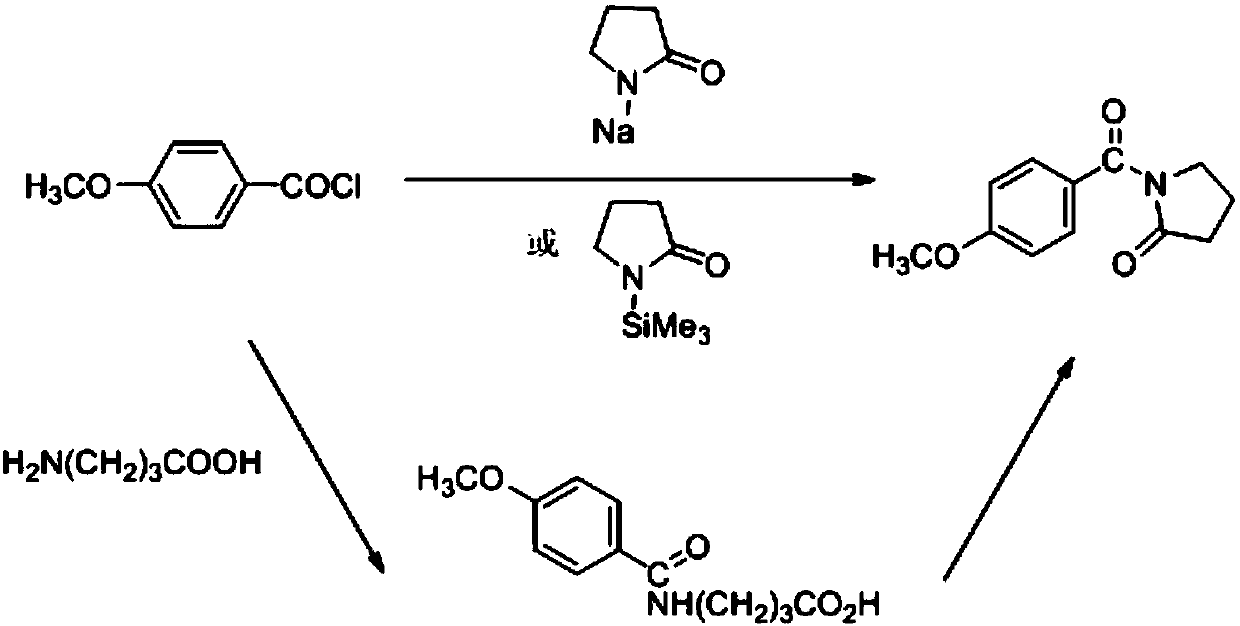
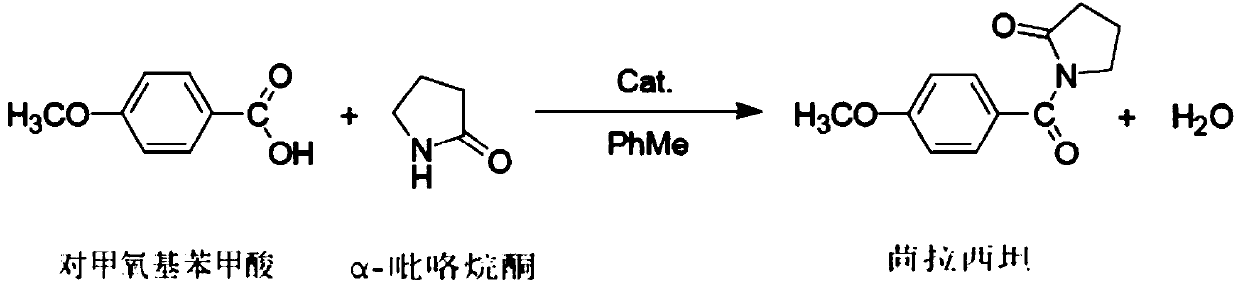
The present invention relates to medicinal chemistry field, be specifically related to a kind of preparation method of aniracetam, and its synthetic route is as follows:
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
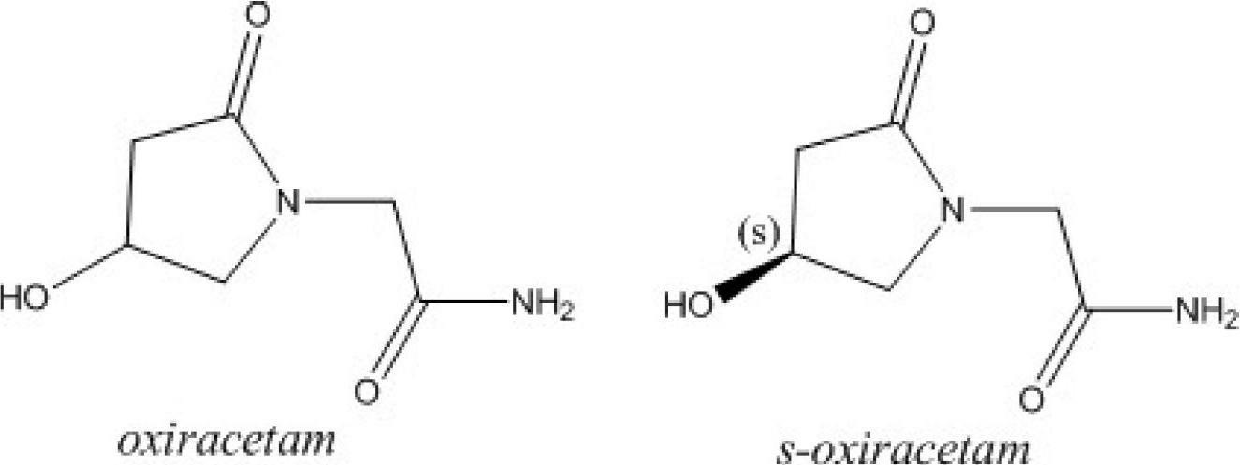
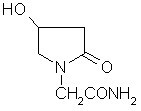
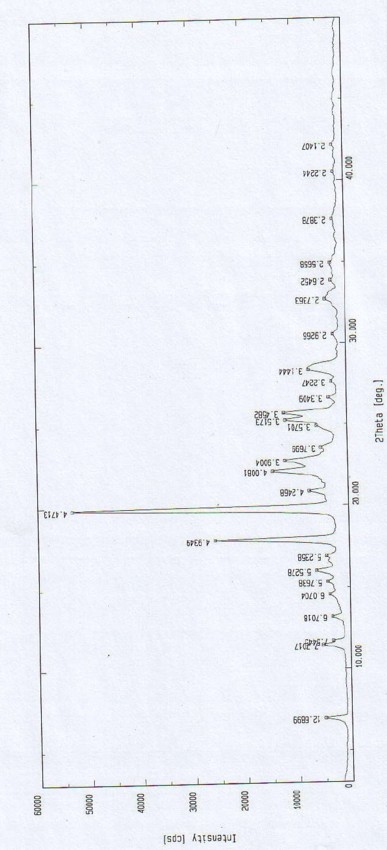
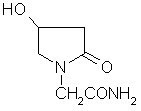
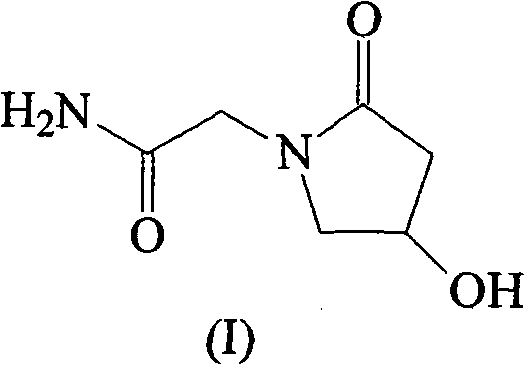
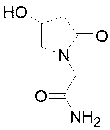
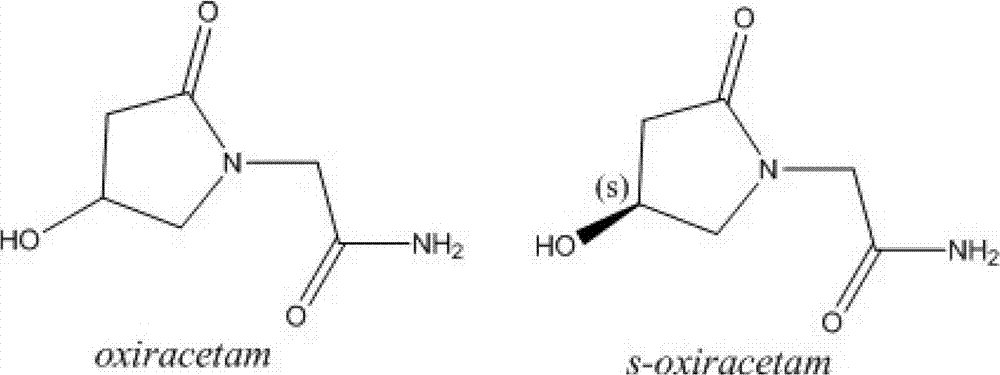
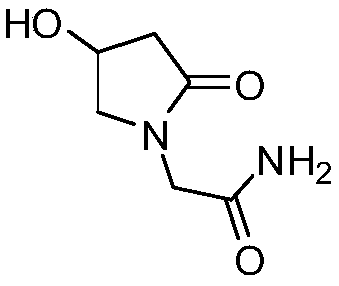
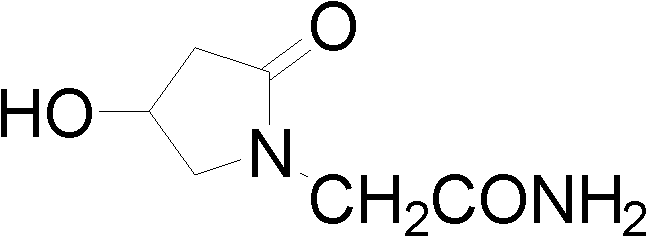
(S)-4-hydroxy-2-oxo-1-pyrrolidineacetamide racemate crystal form II and preparation method therefor
ActiveUS9126929B2High purityConvenient medical treatmentOrganic chemistry1-pyrrolidineacetamidePyrrolidine
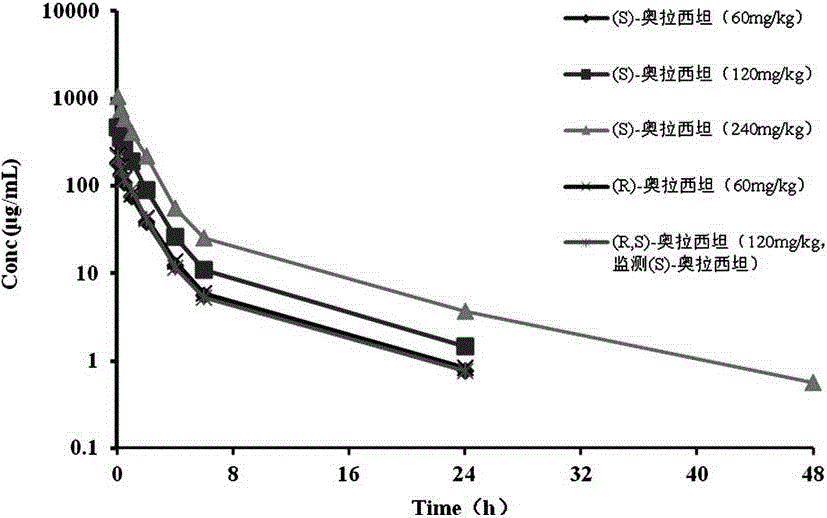
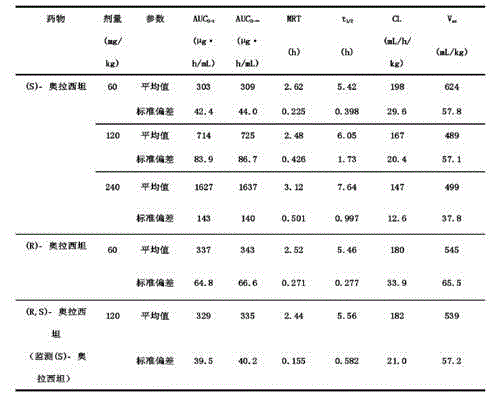
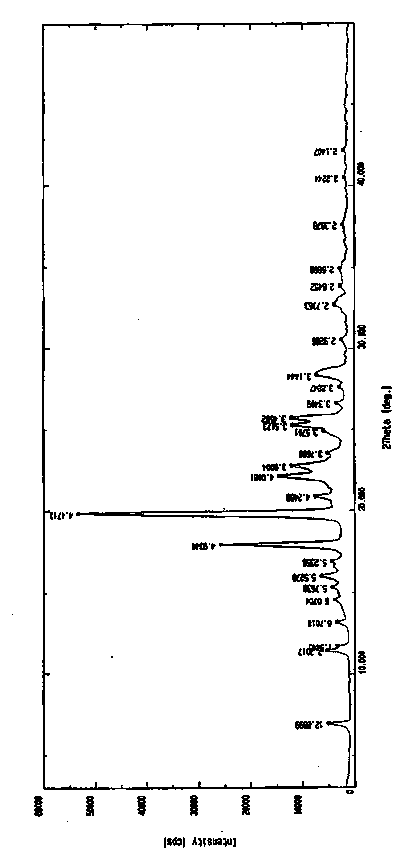
An (S)-4-hydroxy-2-oxo-1-pyrrolidine acetamide racemate referred to as (S)-oxiracetam crystal form II has a diffraction peak at a diffraction angle 2θ of 10.669, 13.25, 13.847, 14.198, 16.729, 17.934, 18.746, 18.816, 20.273, 20.413, 21.431, 21.617, 21.663, 23.38, 24.324, 24.415, 26.069, 26.107, 27.901, 28.621, 28.925, 29.449, 29.484, 31.702, 36.516, 37.685, or 39.721 degrees. The purity of the (S)-oxiracetam crystal form II can be up to 98.5%, and the (S)-oxiracetam crystal form II has the advantages of simple preparation method, mild control condition, low production cost, and the produced oxiracetam hydrate crystal form II has a high purity (the oxiracetam hydrate crystal form having a purity of 8%˜98.5% can be prepared by a crude levo-oxiracetam having a purity of 92%, and thus having a good reproducibility in production.
Owner:CHONGQING RUNZE PHARM CO LTD
Pramiracetam hydrate crystal and preparation method thereof
The invention discloses a pramiracetam hydrate crystal and a preparation method thereof, and belongs to the field of medicines. The preparation method of the pramiracetam hydrate crystal comprises thefollowing steps: providing a pramiracetam solution in which a solvent is an organic reagent; dropwise adding water into the solution, cooling the solution to crystallize, and filtering the liquid; leaching the crystal by using an organic reagent and carrying out suction filtration at the same time; and drying the filter cake under a vacuum condition until the water content is 5-7% to obtain a monohydrate crystal. According to the preparation method, relatively high crystal yield and purity can be obtained.
Owner:ZHEJIANG LANGHUA PHARMA
Preparation method of piracetam freeze-dried powder injection for injection
InactiveCN111888338AImprove quality and stabilityImprove stabilityPowder deliveryOrganic active ingredientsActivated carbonPenicillin
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of a piracetam freeze-dried powder injection for injection. The preparation method of the piracetam freeze-dried powder injection for injection comprises the following steps: (1) putting 60% of water for injection based on the total volume into a mixing tank, adding piracetam in a stirring state, fully dissolving, adding the rest volume of water for injection, stirring, regulating the pH value, adding activated carbon for injection, stirring, filtering, decarbonizing, and continuously stirring, finely filtering, filling into a penicillin bottle, and performing semi-corking; S2) performing pre-freezing on the penicillin bottle in the step S1, starting a condenser, starting a vacuum pump when the temperature of the condenser is reduced to -40 DEG C or below, increasing the temperature to -18 DEG C, and keeping the temperature until the water line disappears; continuously raising the temperature to 35 DEG C, keeping the temperature, pumping out air, filling high-purity nitrogen, and pressing a penicillin bottle plug; and S3) rolling an aluminum-plastic cover, carrying out lightinspection, and packaging to obtain the injection. The piracetam freeze-dried powder injection for injection disclosed by the invention has the advantages that the appearance conforms to the specification, the redissolution is easy, the impurities are few, and the quality is stable.
Owner:广东鼎信医药科技有限公司
Compound preparation of piracetam and its use
InactiveCN1679611AImprove functional statusShort reaction timeOrganic active ingredientsNervous disorderDiseaseHigh energy
A high-energy composite medicine for treating acute or chronic cerebrovascular disease, cerebral trauma, toxipathic cerebrosis, hypomneis and cerebral disfunction contains proportionally inosine, ATP, VC and piracetam.
Owner:FUKANGREN BIO PHARMA
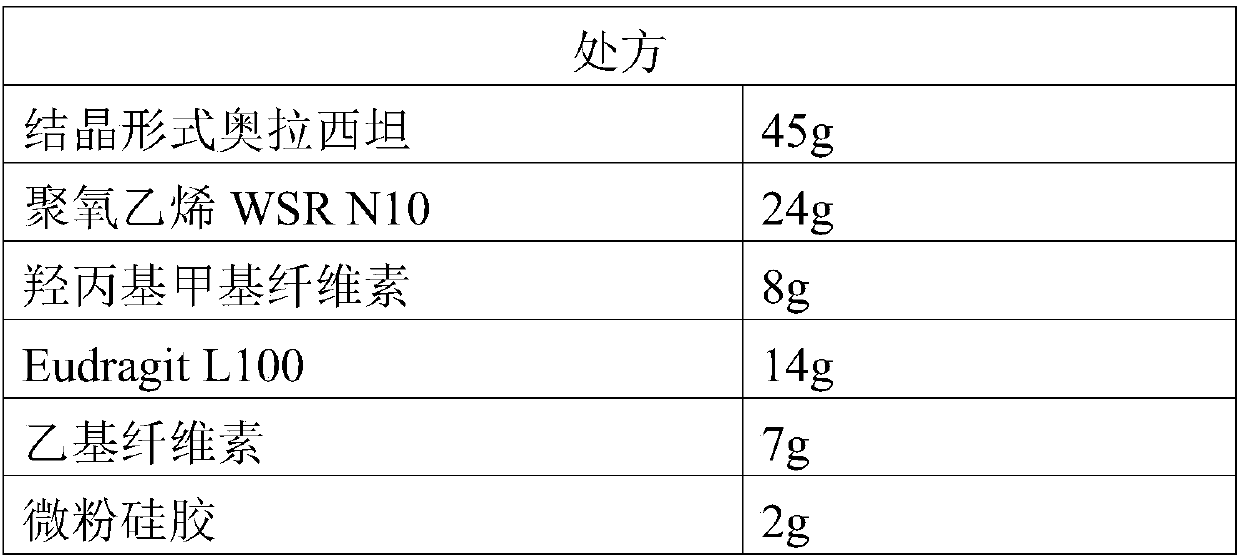
Oxiracetam enteric-coated preparation and preparation method thereof
InactiveCN109718222ALow dissolution rateGood storage stabilityOrganic active ingredientsNervous disorderEnteric-coated granulesDissolution
The invention provides an oxiracetam enteric-coated capsule. Oxiracetam in special crystallization form is used as an active component. A composite carrier containing polyoxyethylene PEO and hydroxypropyl methyl cellulose ( or polyvinylpyrrolidone) in the mass ratio of the polyoxyethylene PEO to the hydroxypropyl methyl cellulose being (1-3) to 1, is carefully selected, and the prepared oxiracetamenteric-coated preparation can reduce the dissolution rate of the active component in the stomach, and also has favorable slow control slow release properties and storage stability. The prepared oxiracetam enteric-coated granules are subjected to long-term stability test under the condition that the temperature is 25 DEG C+ / -2 DEG C, and the relative humidity is 60%+ / -10%, and after long-term experiment for 24 months, sample properties, content and related substances all conform to specification. The oxiracetam enteric-coated capsule is good in stability, and long in quality guarantee period.The preparation method is simple, and suitable for industrial production.
Owner:CHONGQING RUNZE PHARM CO LTD
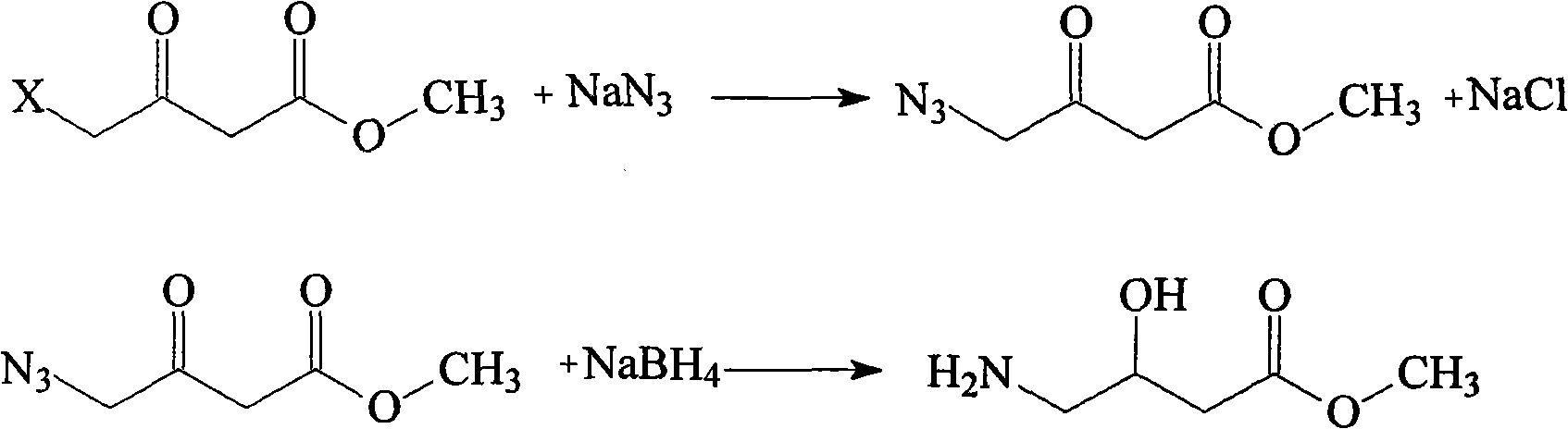
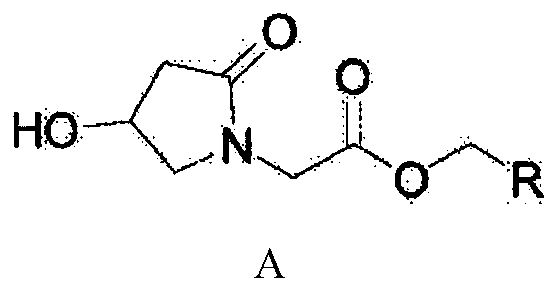
Preparation method of oxiracetam
Belonging to the technical field of medicine, the invention in particular relates to a preparation method of oxiracetam or pharmaceutically acceptable solvates thereof. The method includes the steps of: reacting oxiracetam acid with a compound R-CH2-OH to generate a compound with a structure shown as formula (I) in the specification, wherein R is defined as the specification. The invention also relates to the compound with a structure shown as formula (I) and application thereof in preparation of oxiracetam or pharmaceutically acceptable solvates thereof. The invention also relates to the use of R-CH2-OH in preparation of oxiracetam or pharmaceutically acceptable solvates thereof.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Freeze-dried powder injection of L-oxiracetam and process for preparing freeze-dried powder injection
ActiveCN102670527BFast sublimationShorten freeze-drying timeOrganic active ingredientsPowder deliveryFreeze-dryingPolyethylene glycol
The invention relates to a freeze-dried powder injection of L-oxiracetam and a process for preparing the freeze-dried powder injection. The freeze-dried powder injection comprises, by weight, 1 part of the L-oxiracetam, 0.1 to 10 parts of an excipient, 0.01 to 1 part of an antisticking agent and 0.01 to 0.5 part of a pH regulator, wherein polyethylene glycol (PEG) series of products serve as the antisticking agent preferably. The freeze-dried powder injection which is prepared on the basis of a prescription according to the process has the advantages of being short in freeze-drying time, attractive in appearance, short in redissolving time and high in stability.
Owner:南京博德生物制药有限公司
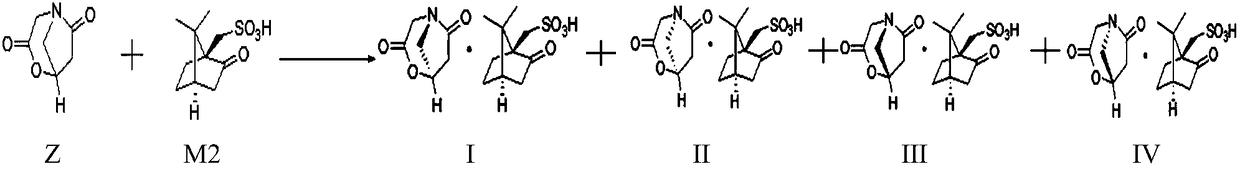
Preparation method of oxiracetam isomer
The invention belongs to the field of drug synthesis, and particularly relates to a preparation method of oxiracetam isomer. (S)-oxiracetam and (R)-oxiracetam are prepared by the following steps: 1) dehydrating in an oxiracetam acid molecule to generate oxiracetam lactone; 2) enabling lactone to react with D(+)-10-camphorsulfonic acid to generate (1R, 5R), (1S, 5R), (1R, 5S) and (1S, 5S) types ofoxiracetam acid lactone salt; 3) enabling (1R, 5R) and (1S, 5R) types of oxiracetam acid lactone salt to react with ammonia to geneate (R)-oxiracetam, and enabling (1R, 5S) and (1S, 5S) types of oxiracetam acid lactone salt to react with ammonia water to generate (S)-oxiracetam. The method provided by the invention can be used for preparing (S)-oxiracetam and (R)-oxiracetam. The method provided bythe invention can be used for preparing (S)-oxiracetam and (R)-oxiracetam, does not waste materials, has the advantages of simple operation, high product yield (up to 60%) and high product purity (99.8% or higher), and is suitable for industrialized large-scale production.
Owner:福安药业集团重庆博圣制药有限公司
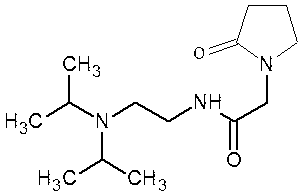
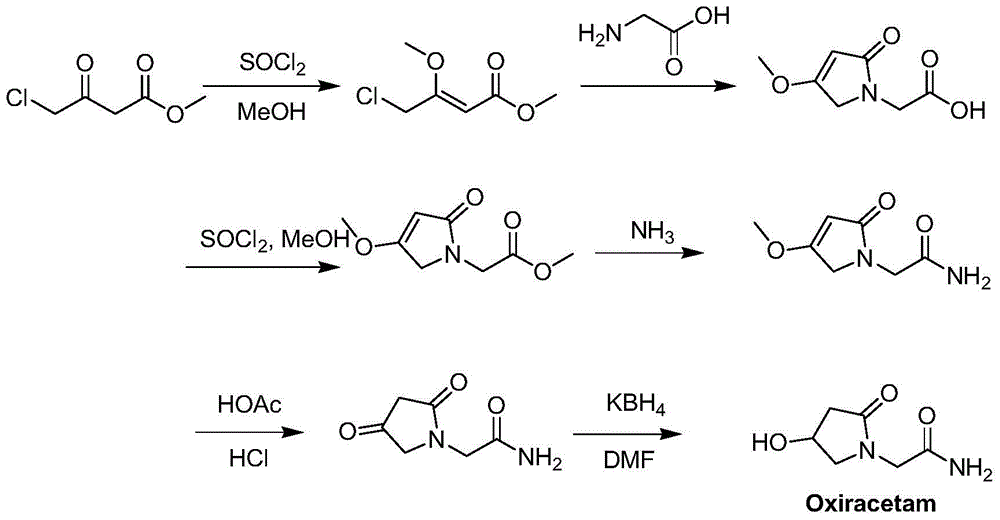
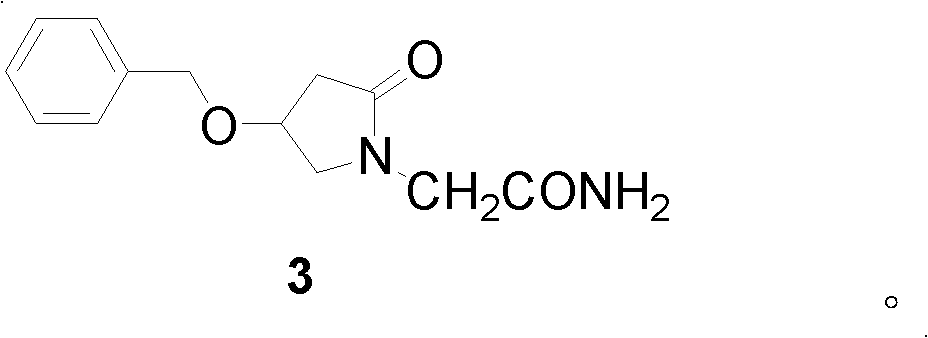
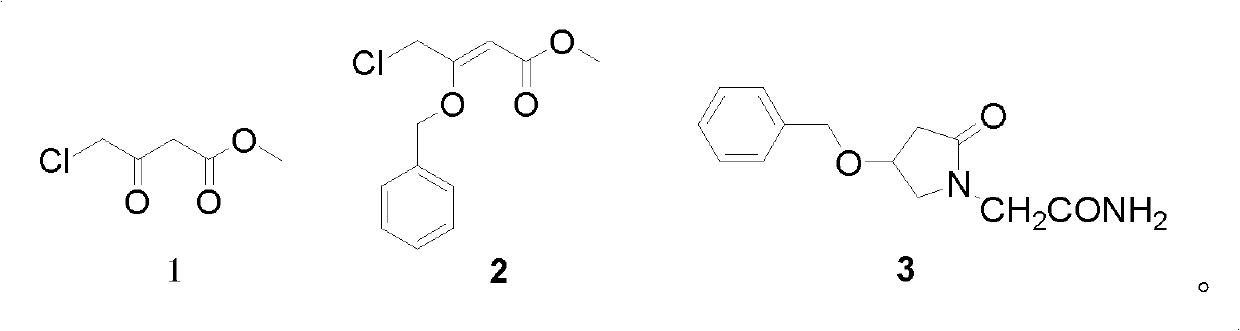
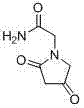
2-(4- OBzl-2-oxo-2,5-pyrroline-1-yl)-acetamide and synthesis and application thereof
The invention discloses 2-(4- OBzl-2-oxo-2,5-pyrroline-1-yl)-acetamide and synthesis and application thereof. The structure of 2-(4- OBzl-2-oxo-2,5-pyrroline-1-yl)-acetamide is indicated as the formula (3), and the synthesis route is indicated as follows. 2-(4- OBzl-2-oxo-2,5-pyrroline-1-yl)-acetamide can serve as an intermediate to be used for synthesis of oxiracetam, has the advantages that synthesis steps are short, operation is simple and convenient, raw materials are cheap and easy to obtain and emission of three wastes is little, and enjoys broad industrial application prospects.
Owner:HANGZHOU NORMAL UNIVERSITY
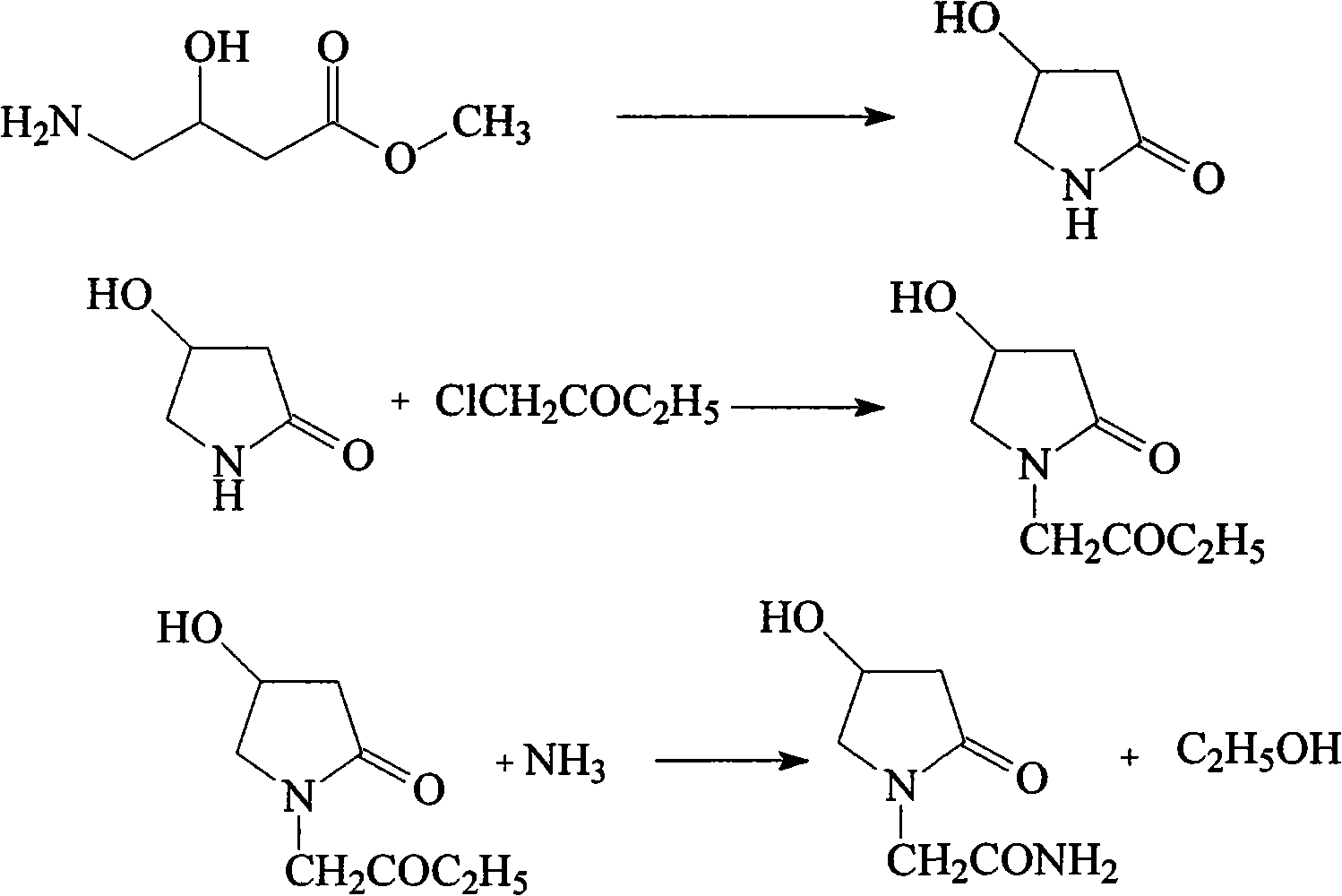
Novel synthesis process for oxiracetam key intermediate 2-(2,4-dioxopyrrolidine-1-yl)-acetamide
The invention provides a novel synthesis process for preparing an oxiracetam key intermediate 2-(2,4-dioxopyrrolidine-1-yl)-acetamide by using 4-halogenated acetoacetic ester and glycinamide hydrochloride as raw materials by virtue of a 'one-pot method'. The novel synthesis process has the advantages of being few in reaction steps, high in yield, cheap and easily available in raw materials, simple in post-treatment, has few waste gas, waste water and waste residues and the like.
Owner:ZHEJIANG UNIV OF TECH
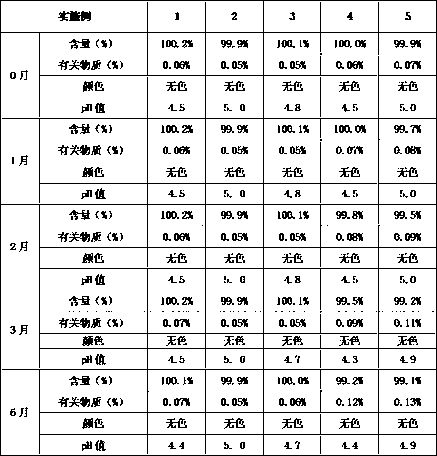
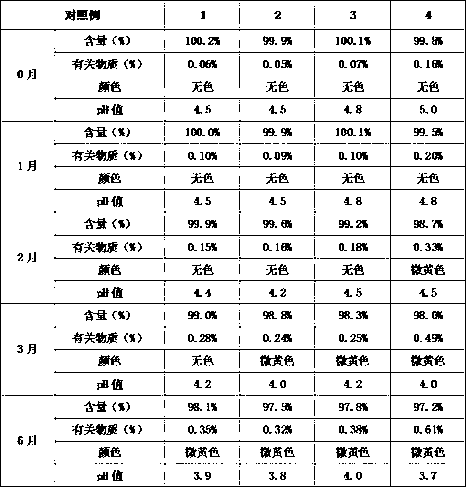
Racetam pharmaceutical composition containing buffering agent
ActiveCN107753481AInexpensive and safeSolve the problem of high temperature resistanceOrganic active ingredientsNervous disorderSodium acetateMANNITOL/SORBITOL
The invention belongs to the technical field of medicine, and relates to a racetam pharmaceutical composition containing buffering agent. The composition is prepared from the buffering agent, racetammedicine, mannitol and water, and is characterized in that the total massic volume percentage of racetam medicine and mannitol is 10-60% every 100 mL. The buffering agent is composite buffer salts ofone or two types of phosphate, acetate and citrate. The composition with addition of monosodium phosphate-dilute hydrochloric acid and sodium acetate-dilute hydrochloric acid has the optimal stability, the pH value of the buffering agent is 4.50-6.50, and the amount of the buffer salts is 0.5-1.0 mmol every 100 mL liquid. According to the racetam pharmaceutical composition containing the bufferingagent, through addition of the buffering agent into the composition, the stability is improved, the problem that the pH value and the relevant substance content of the racetam medicine significantlychange is solved, and the composition has broad application prospects.
Owner:SHENYANG PHARMA UNIVERSITY
Oxiracetam compound with steady crystal form
The invention belongs to the technical field of medicine, and particularly relates to an oxiracetam compound. The oxiracetam compound provided by the invention contains semi-crystalline water. The oxiracetam compound has the advantages that related substances are small, the purity is high, the stability is good, and a moisture-absorption and weight-gaining effect is not obvious even if under a high humidity condition.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Stable and safe oxiracetam pharmaceutical composition for injection
InactiveCN102512378BSolve discolorationAvoid elevationPowder deliveryOrganic active ingredientsSide effectTherapeutic effect
The invention relates to a stable and safe oxiracetam pharmaceutical composition for injection and a preparation method thereof, especially to an oxiracetam pharmaceutical composition for treating brain damage and neurological deficit caused by the brain damage as well as impediment of anamnesis and aptitude. Preferred are injections and lyophilized powders. The oxiracetam pharmaceutical composition provided by the invention mainly contains active components of oxiracetam, sodium chloride and citric acid. The oxiracetam pharmaceutical composition for injection has stable pH values and low impurity content. By the adoption of the preparation method, curative effects of the product are effectively raised, side effects are minimized, and simultaneously the yield is raised, cost is reduced, and higher benefits are created.
Owner:TIANJIN HANRUI PHARMA
Oxiracetam compound
ActiveCN104370792ASuitable for manufacturingSuitable for long term storageOrganic active ingredientsNervous disorderCerebral damagePharmaceutical drug
The invention belongs to the technical field of medicine, and specifically relates to an oxiracetam compound and a preparation method thereof. The provided oxiracetam compound contains a half of crystal water, and has the advantages of high purity, good stability, and excellent technology repeatability. The invention also relates to an application of oxiracetam in preparation of drugs for treating brain damages caused by physical and chemical factors, various cerebral anoxia, and chronic cerebral insufficiency.
Owner:TIANJIN HANRUI PHARMA
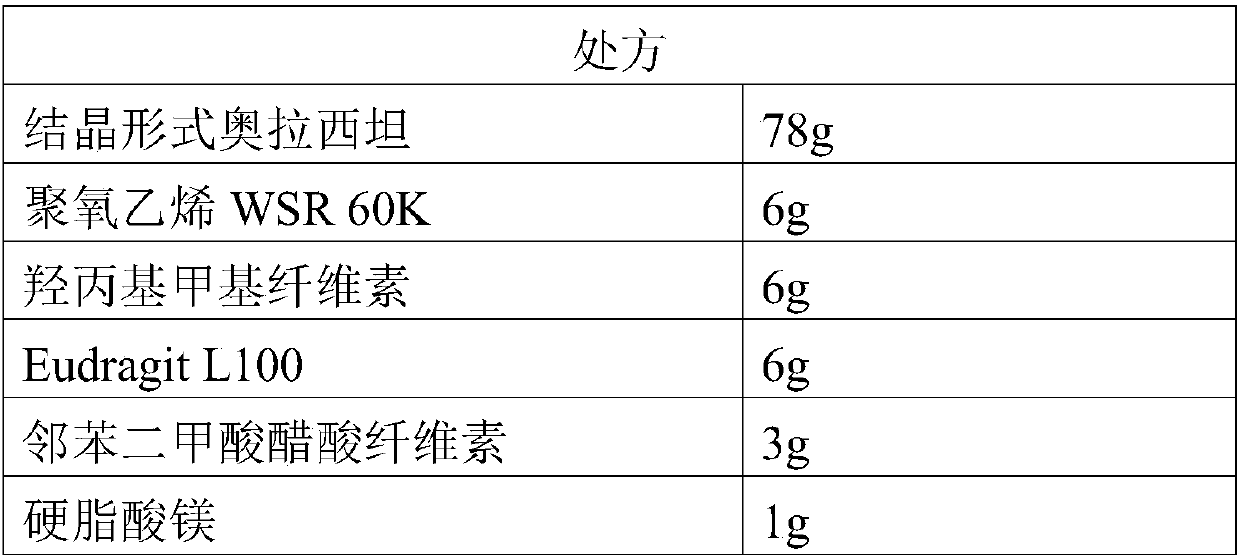
Dextro-oxiracetam sterile powder for injection and preparation method thereof
InactiveCN110384660AReduce humidityImprove stabilityPowder deliveryOrganic active ingredientsArginineMedicinal chemistry
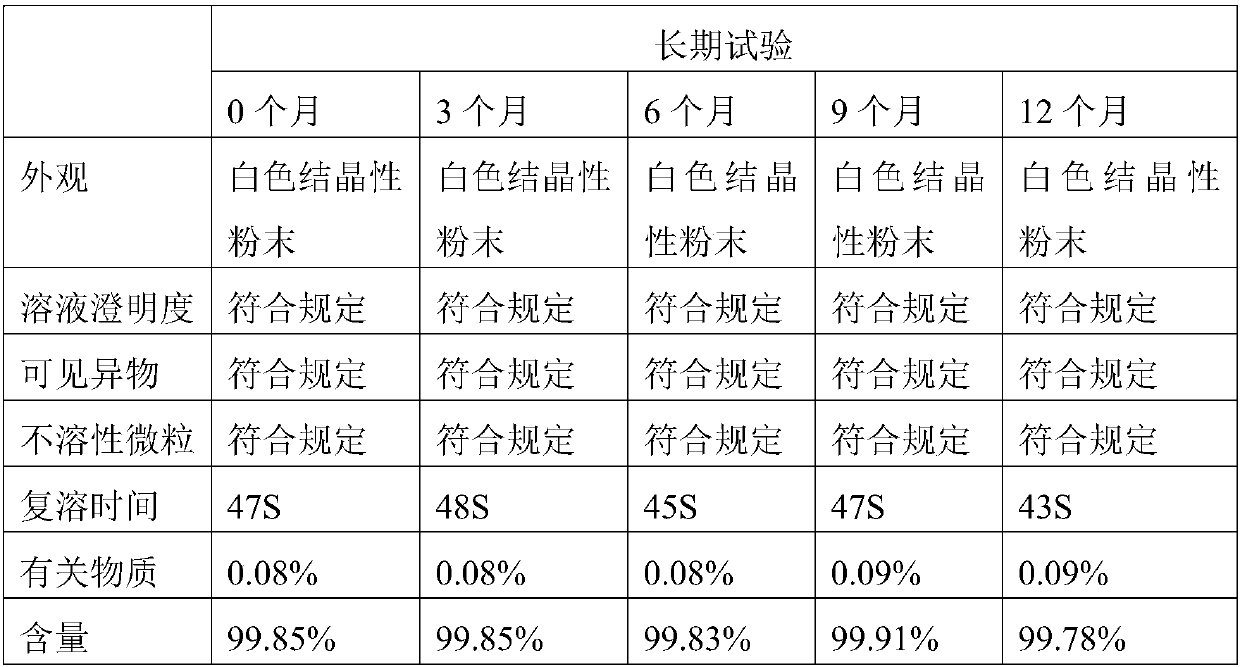
The invention provides dextro-oxiracetam sterile powder for injection. The dextro-oxiracetam sterile powder is prepared from dextro-oxiracetam in a crystal form and L-arginine in a specific proportion, wherein the dextro-oxiracetam has diffraction peaks when the reflection angle 2*theta is 10.54+ / -0.2 degrees, 13.76+ / -0.2 degrees, 14.14+ / -0.2 degrees, 16.64+ / -0.2 degrees, 17.76+ / -0.2 degrees, 18.72+ / -0.2 degrees, 20.16+ / -0.2 degrees, 21.20+ / -0.2 degrees, 21.52+ / -0.2 degrees, 23.25+ / -0.2 degrees, 24.17+ / -0.2 degrees, 25.88+ / -0.2 degrees, 27.61+ / -0.2 degrees, 28.57+ / -0.2 degrees, 29.24+ / -0.2 degrees and 31.40+ / -0.2 degrees; and hygroscopicity is low, stability is good, and the quality of the dextro-oxiracetam sterile powder is kept advantageously. Under the commercially available back condition, the dextro-oxiracetam sterile powder is subjected to an acceleration test for 6 months at 35+ / -5 DEG C and subjected to a long-term test for 12 months at the room temperature to be investigated,all indexes are not changed remarkably and are within the range prescribed by the quality standard, and the stability is good; and a preparation process is simple, short in operation time, high in production efficiency and suitable for industrial production.
Owner:CHONGQING RUNZE PHARM CO LTD
Industrial preparation method of pramiracetam sulfate
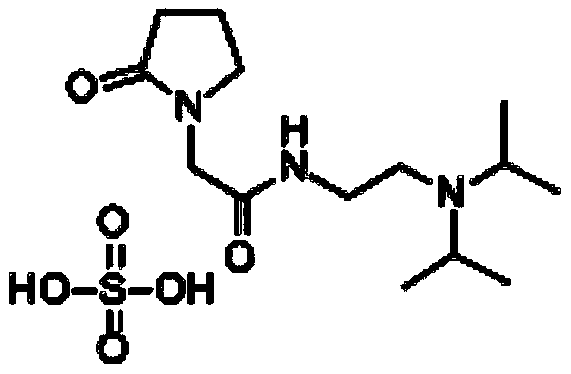
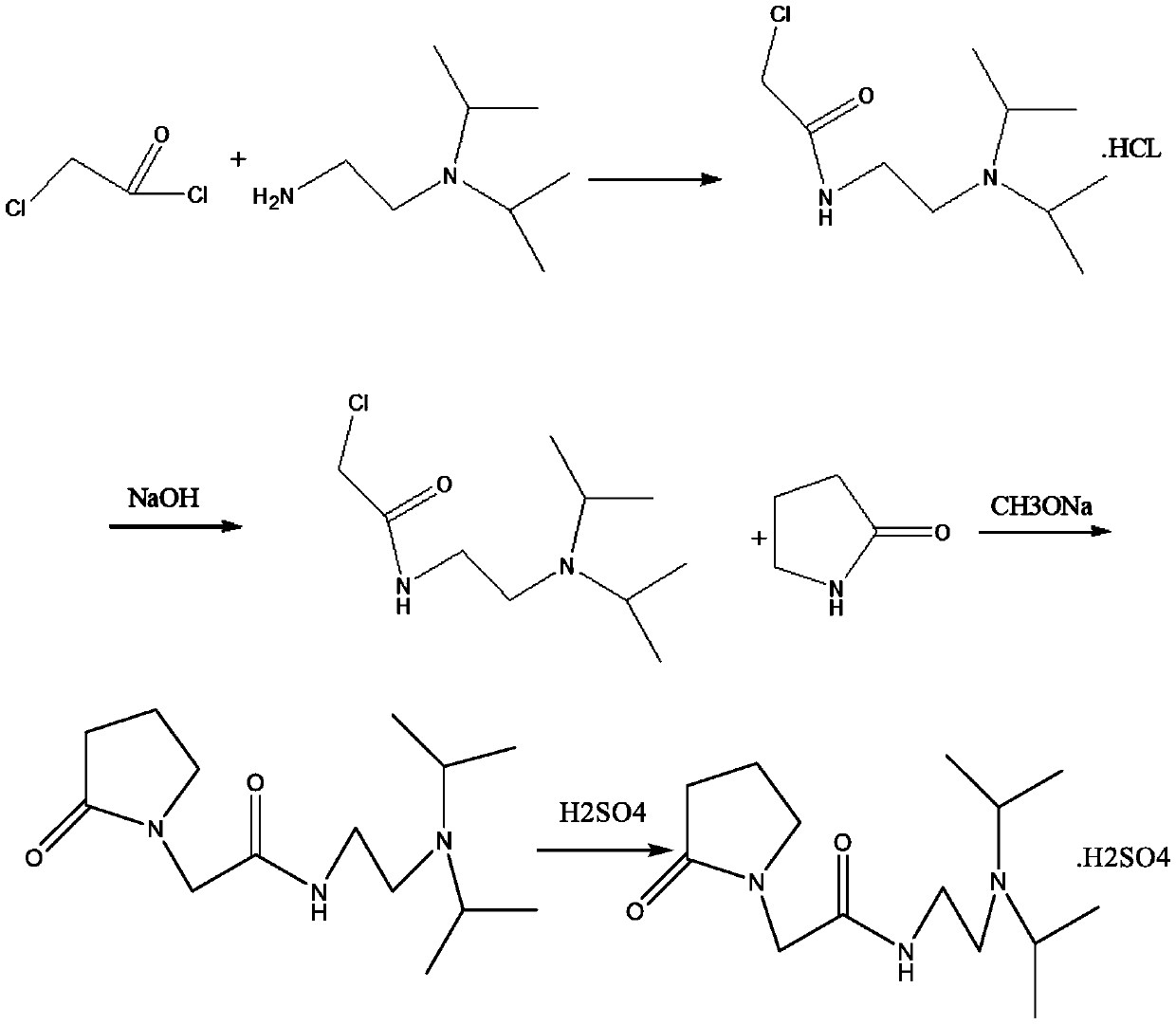
The invention relates to the field of preparation of compounds, in particular to an industrial preparation method of pramiracetam sulfate. The method includes: (1) converting N-(2-(diisopropyl)ethyl)-2-chloroacetamide hydrochloride to obtain free amide, and removing moisture in the free amide; (2) reacting solid alkali with alpha-pyrrolidone in a solvent I under the protection of nitrogen, simultaneously distilling alcohol generated by the reaction, then adding a solution prepared from the free amide and the solvent I dropwise, and carrying out reaction to obtain pramiracetam; and (3) preparing pramiracetam and sulfuric acid into pramiracetam sulfate. According to the method, pramiracetam sulfate with excellent quality can be efficiently obtained, and the method has the characteristics oflow cost, high environmental friendliness, no use of dangerous materials, and high process safety, and is beneficial to popularization and application in industrial preparation.
Owner:BEIJING YUEKANGKECHUANG PHARM TECH CO LTD
Oxiracetam lipid micro-bubble and preparation method thereof
InactiveCN105560230AImprove permeabilityAchieve targeted drug deliveryPowder deliveryOrganic active ingredientsChemical reactionPhospholipid
The invention provides an oxiracetam lipid micro-bubble. The oxiracetam lipid micro-bubble comprises a phospholipid bi-layer membrane, a gas and an oxiracetam-supported alhumin nanoparticle, wherein the gas and the oxiracetam-supported alhumin nanoparticle are wrapped in the phospholipid bi-layer membrane, and the gas is a nitrogen and perfluoropropane mixed gas with the volume ratio of 1:2-5. The oxiracetam lipid micro-bubble has high drug loading capacity and entrapment rate, can improve the plasma concentration of a target position, prolongs the action time of drugs in the target position, reduces drug degradation and improves the drug stability, and the nanoparticle can promote selective distribution of the drugs, facilitation of delivery of the drugs to lesion tissues, increases the drug effects and reduces toxic and side effects. The invention also discloses a preparation method of the lipid micro-bubble. The preparation method has the advantages of simplicity, easy industrialization, no involving of chemical reactions in the preparation process, no use of highly toxic organic solvents, high safety and strong environmental protection property.
Owner:CHONGQING RUNZE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com