Racetam pharmaceutical composition containing buffering agent
A buffer and composition technology, applied in the field of medicine, can solve problems such as non-conformity, inability to apply clinically, and quality changes, and achieve the effect of solving high temperature resistance, low price, and safe price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: Stability research test
[0038] Prepare the racetam drug composition: the total mass volume percentage of the racetam drug and mannitol is in the range of 20% to 30%, place it at room temperature (25°C) and 4°C, and observe the crystallization state after 10 days; at the same time Another batch was frozen at -20°C for 2 days, and then placed at 40°C for 2 days to observe the reconstitution state. The results are shown in Tables 2-4.
[0039] The racetam pharmaceutical composition and mannitol described in the present invention can be prepared by the following method, taking piracetam as an example.
[0040] Dissolve piracetam and mannitol in the prescribed amount according to Table 1, dissolve in sterile water for injection, add 0.5% (w / v) activated carbon, absorb at 100°C for 30min, filter while hot, potting, and sterilize at 121°C for 8min .
[0041] Table 1 Basic Prescription
[0042]
[0043] The control group is a mannitol solution with a conce...
Embodiment 2
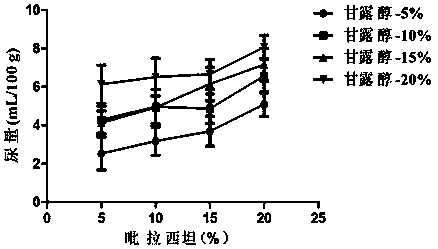
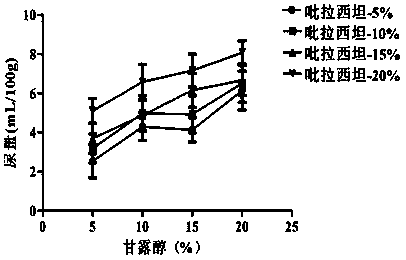
[0059] Embodiment 2: diuresis test
[0060] For acute diseases, such as acute cerebral edema induced by various etiologies, immediate dehydration is required. At this time, the combination of piracetam and mannitol is required to exert its high efficacy. The inventor selected several groups of compositions with different concentrations and carried out Diuretic test, the specific operation process is as follows:
[0061] Male Wistar rats, weighing 200±20g, were randomly divided into 18 groups, with 3 rats in each group, placed in metabolic cages for 1 day to adapt to the environment, and observed whether the urine output was stable under the condition of free drinking water. No food or water was allowed 18 hours before the experiment. Following the diuretic screening method introduced by Aston, 2.2mL / 100g distilled water was administered into the stomach before the drug to maintain a certain water load in the animal body. Urine was collected for 2 hours, and those whose urine...
Embodiment 3
[0067] Embodiment 3: sodium acetate-dilute hydrochloric acid buffer solution
[0068] From Example 1, the mass volume ratio of piracetam to mannitol is 10:15 as an example, and the basic prescription is shown in Table 7.
[0069] Table 7 Basic Prescription
[0070]
[0071] Sodium acetate-dilute hydrochloric acid buffer preparation method: Weigh 1.64g sodium acetate into a 100mL measuring bottle, add sterile injection solution to dissolve in water and dilute to the mark, adjust the pH to 3.00, 3.50, 4.00, 4.50, 5.00, 5.50, 6.00, 6.50, 7.00 to make sodium acetate-dilute hydrochloric acid buffer solutions with different pH values.
[0072] Preparation method: Dissolve piracetam and mannitol in the prescribed amount according to Table 7, dissolve in sterile water for injection, add 0.5% (w / v) activated carbon, absorb at 100°C for 30min, filter while hot, add buffer, and stir Uniform, potted, sterilized at 121°C for 8 minutes, measured the pH value before and after sterilizat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com