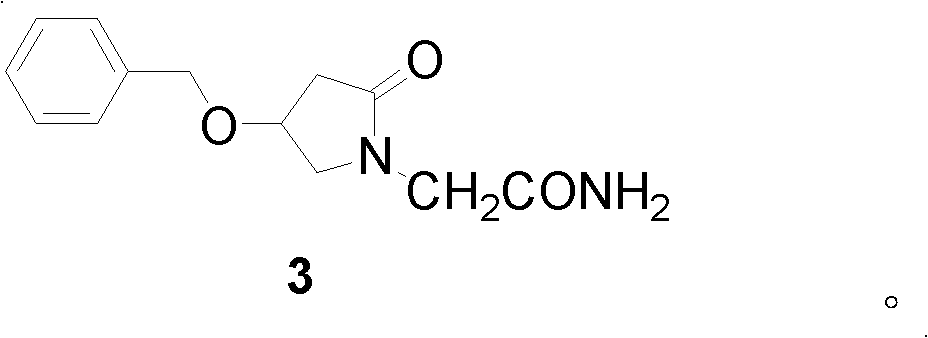
2-(4- OBzl-2-oxo-2,5-pyrroline-1-yl)-acetamide and synthesis and application thereof
A technology of dihydropyrrole and benzyloxy, which is applied in the application field of key intermediates, can solve the problems that purification cannot meet industrial production requirements, complicated operation, low total yield, etc., and achieves good industrial application prospects and cheap and easy raw materials. The effect of high yield and product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
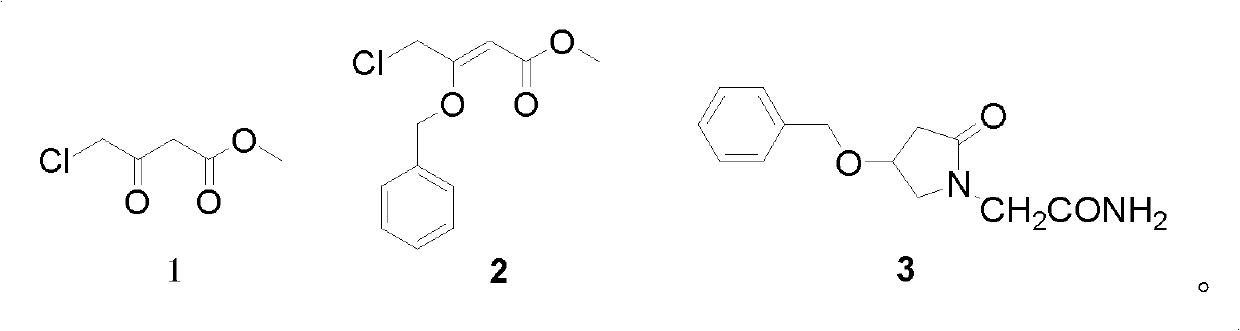
[0037] Example 1 Synthesis of 3-benzyloxy-4-chloro-2-butenoic acid methyl ester.
[0038] Method 1: Take a dry 100mL three-neck round bottom flask, add 1.5g (0.01mol) methyl 4-chloroacetoacetate, 1.08g (0.01mol) benzyl alcohol and 45mg p-toluenesulfonic acid, add 30mL Cyclohexane, magnetic stirring, oil bath heating, water separator reflux, reaction for 5h, cooling to room temperature, desolvation under reduced pressure, to obtain a light yellow oily liquid, which is 3-benzyloxy-4-chloro-2-butene Acid methyl ester, gas phase yield 15.20%, directly used in next step reaction.
[0039] Method 2: Take a dry 100mL three-neck round bottom flask, add 1.5g (0.01mol) methyl 4-chloroacetoacetate, 1.08g (0.01mol) benzyl alcohol and 5mL aluminum chloride solution, add 30mL Cyclohexane, magnetic stirring, oil bath heating, water separator reflux, reaction for 5h, cooling to room temperature, desolvation under reduced pressure, to obtain a light yellow oily liquid, which is 3-benzyloxy-4-...
Embodiment 2
[0043] Example 2 Synthesis of 2-(4-benzyloxy-2-oxo-2,5-dihydropyrrol-1-yl)-acetamide 2.
[0044] Method 1. 2.40g (about 0.01mol) of 3-benzyloxy-4-chloro-2-butenoic acid methyl ester prepared in Example 1, 1.12g (0.01mol) glycinamide hydrochloride and 30mL methanol Put the solvent in a 100mL three-necked flask, add 10mol / L sodium carbonate solution dropwise under stirring, keep the pH of the system between 8-9, stir magnetically for 14 hours at a temperature of 60°C, then add 15mL of distilled water, stir and filter with suction , a yellow viscous crude product was obtained. The crude product was recrystallized several times with a recrystallization solvent to obtain a yellow powdery solid, GC-MS: m / z=246[M] + And there is only one peak, the yield is 70.26%. Melting point: 247.2-248.6°C.
[0045] Method 2. 2.40g (about 0.01mol) of 3-benzyloxy-4-chloro-2-butenoic acid methyl ester prepared in Example 1, 1.12g (0.01mol) glycinamide hydrochloride and 30mL methanol Put the solv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com