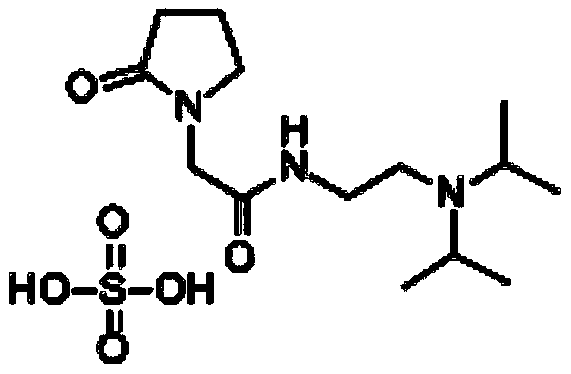
Industrial preparation method of pramiracetam sulfate
A technology of pramiracetam sulfate and hydrochloride, which is applied in the field of compound preparation, can solve the problems of difficult filtration, unsafe process, incomplete reaction, etc., and achieve improved yield, process stability, high process safety, The effect of avoiding generation of water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
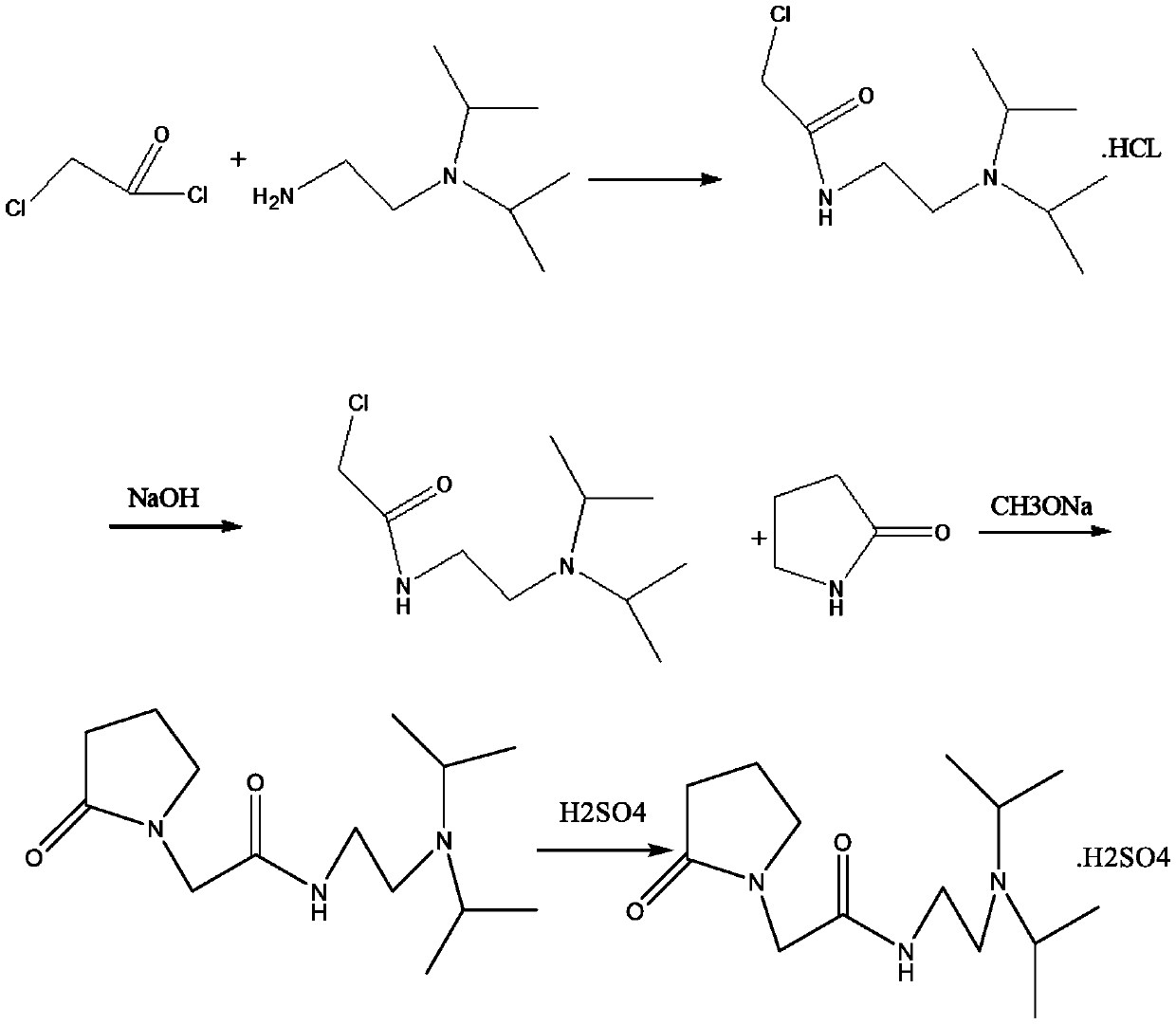
[0053] This embodiment provides an industrialized preparation method of pramiracetam sulfate, wherein the molar ratio of the free amide, α-pyrrolidone and sodium methoxide is about 1:1.5:1.5, and the specific process is as follows:
[0054] (1) Add 3.6 liters of tetrahydrofuran, 474.4 grams of diisopropylethylenediamine, cool down to zero degrees, dropwise add 421.1 grams of chloroacetyl chloride dissolved in 400mlTHF, drop it at 15 degrees for one hour and forty minutes, and keep warm at 10 degrees to react 3h, filtered, rinsed with a small amount of THF, and air-dried at 60 degrees to obtain 821.0 grams of material (slightly yellow), with a yield of 97.06% and a purity of 96.8%, heated and dissolved with 1.65 liters of absolute ethanol to absorb 50 grams of activated carbon, filtered while hot, Crystallized at 0-5 degrees, filtered and dried to obtain 650.0 g of N-(2-(diisopropyl)ethyl)-2-chloroacetamide hydrochloride, with a yield of 79.17% and a purity of about 99%.
[005...
Embodiment 2
[0060] The difference between this example and Example 1 is that in step (3), the molar ratio of the free amide, α-pyrrolidone and sodium methylate is adjusted to 1:1.5:2.5, and the specific process is as follows:
[0061] (1) Add 3.6 liters of tetrahydrofuran, 474.4 grams of diisopropylethylenediamine, cool down to zero degrees, dropwise add 421.1 grams of chloroacetyl chloride dissolved in 400mlTHF, drop it at 15 degrees for one hour and forty minutes, and keep warm at 10 degrees to react 3h, filtered, rinsed with a small amount of THF, and air-dried at 60 degrees to obtain 821.0 grams of material (slightly yellow), with a yield of 97.06% and a purity of 96.8%, heated and dissolved with 1.65 liters of absolute ethanol to absorb 50 grams of activated carbon, filtered while hot, Crystallized at 0-5 degrees, filtered and dried to obtain 650.0 g of N-(2-(diisopropyl)ethyl)-2-chloroacetamide hydrochloride, with a yield of 79.17% and a purity of about 99%.
[0062] (2) Dissolve 64...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com