Dextro-oxiracetam sterile powder for injection and preparation method thereof
A technology for sterile powders and injections, which is applied in powder delivery, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc. It can solve problems such as unfavorable drug safety, achieve good clarity and simple preparation process , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
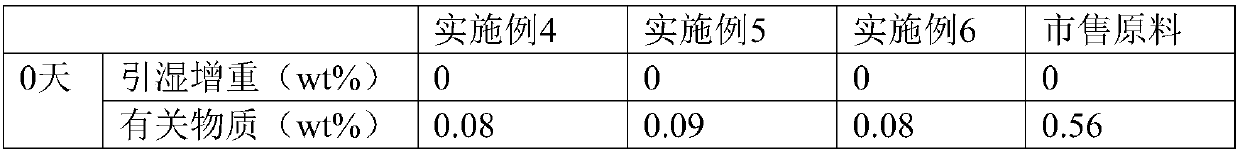
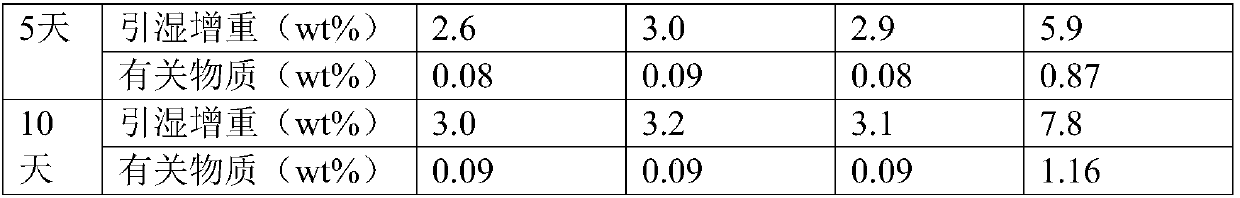
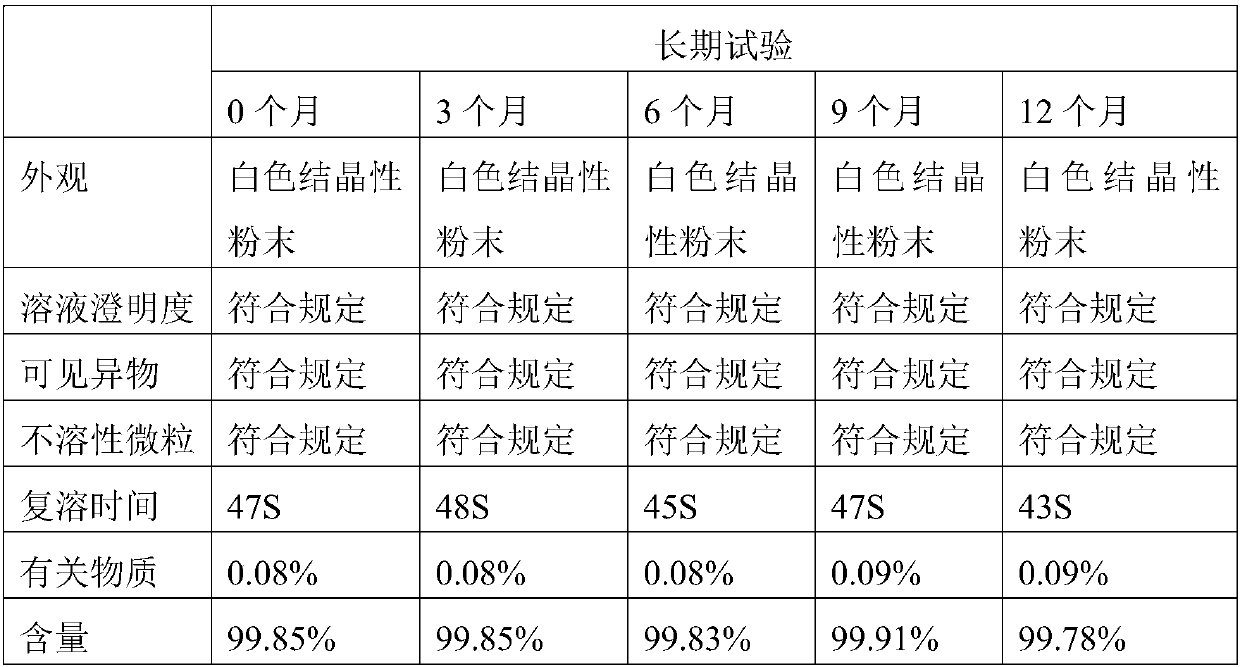
[0019] Dissolve the commercially available dexoxiracetam in formic acid with a mass concentration of 85%, the weight ratio of formic acid to dexoxiracetam is 5:1, keep stirring at a stirring speed of 200r / min, and heat up to 95°C Insulate for 45 minutes, add activated carbon for decolorization, filter, adjust the pH value of the filtrate to 6.0 with 20% sodium hydroxide aqueous solution, concentrate to a supersaturated solution, crystallize at 2°C, filter with suction, collect the filter residue, and store at 30°C, relatively The humidity is 75% and dried for 4 hours.
[0020] Test instrument conditions: Bruker D2 PHASER powder diffractometer is used for normal temperature test, the test conditions are: Cu Ka It is the light source, the voltage is 30kV, the current is 10mA, the test step is 0.014°, the scanning speed is 0.1s / step, and the scanning range is 5-40° (2θ). After testing, the D-oxiracetam crystal prepared in Example 1 has diffraction angles 2θ of 10.54±0.2°, 13.76...
Embodiment 2
[0024] Dissolve 10 g of commercially available Dexoxiracetam in 40 mL of 82% formic acid (w / w), raise the temperature to 90°C and keep it warm for 50 min, then add 0.01 g of activated carbon for decolorization, filter while it is hot, and add 10% hydrogen to the filtrate after it is cooled to room temperature Sodium oxide aqueous solution (w / w) adjusted the pH to 5.5, concentrated into a supersaturated solution, crystallized at 0°C, filtered with suction, collected the filter residue, dried at 35°C for 4 hours at a relative humidity of 85%, and obtained 8.0 g of Oxiracetam crystals. The determination method in Example 1 is used to detect that the crystalline compound of Dexoxiracetam prepared in Example 2 is the same crystal form of Dexoxiracetam in Example 1.
Embodiment 3
[0026] Dissolve 10 g of commercially available Dexoxiracetam in 50 mL of 89% formic acid (w / w), raise the temperature to 110°C and keep it warm for 30 min, then add 0.02 g of activated carbon for decolorization, filter while it is hot, and add 20% hydrogen after cooling the filtrate to room temperature Sodium oxide aqueous solution (w / w) adjusted the pH to 6.5, concentrated into a supersaturated solution, crystallized at 5°C, filtered with suction, collected the filter residue, dried at 25°C for 3 hours at a relative humidity of 70%, and obtained 8.5g Oxiracetam crystals. The assay method in Example 1 was used to detect that the crystalline dexoxiracetam compound prepared in Example 3 was the same crystal form of Dexoxiracetam as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com