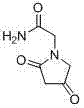
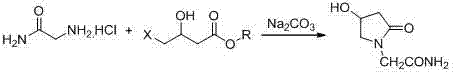
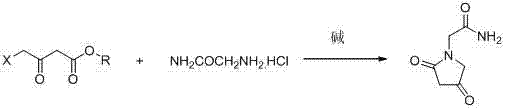Novel synthesis process for oxiracetam key intermediate 2-(2,4-dioxopyrrolidine-1-yl)-acetamide
A technology of dioxopyrrolidine and intermediates, which is applied to the new synthesis process field of oxiracetam key intermediate 2-(2,4-dioxopyrrolidin-1-yl)-acetamide, can solve the problem of Lack of efficient synthesis methods for intermediates, low yields, long synthesis steps, etc., to achieve the effects of good industrial application prospects, high yields, and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0023] In a three-necked flask, add 8.0 g (0.075 mol) glycinamide hydrochloride, 20.2 g (0.029 mol) potassium carbonate, and 100 ml absolute ethanol. Stir at 45°C for 30 minutes, and slowly add 10 g (0.06 mol) dropwise. ) Ethyl 4-chloroacetoacetate, drip and reflux for 5h. , Filtered while hot, removed part of the ethanol under reduced pressure, and the residue was cooled and filtered to obtain 6.7 g of the key intermediate of 2-(2,4-dicarbonylpyrrolidone-1-yl)-acetamide with a yield of 72.0%. 1 H NMR (500 MHz, DMSO- d 6 ) δ 7.67 (s, 1H), 7.49 (s, 1H), 7.22 (s, 1H), 4.65 (s, 2H), 4.48 (s, 1H), 3.67 (d, J = 5.6 Hz, 2H); 13 C NMR (125 MHz, DMSO- d 6 ) δ 174.71 (s), 169.90 (s), 168.97 (s), 79.37 (s), 67.16 (s), 46.90 (s); GC / MS: m / z=156 [M] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com