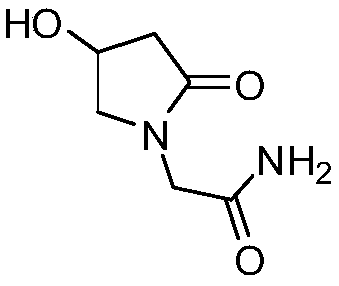
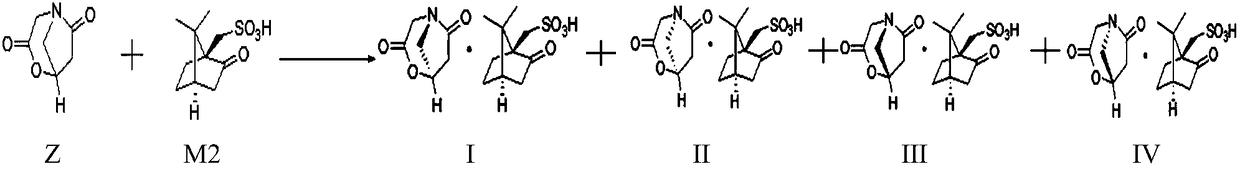
Preparation method of oxiracetam isomer
A technology of isomers and acid lactones, applied in the field of drug synthesis, can solve the problems such as no, inability to distinguish oxiracetam, and limited utilization, and achieve the effects of simple operation, high product yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1 comprises the preparation of the reaction system of oxiracetam acid lactone salt isomer
[0065] 1. Preparation of Oxiracetam Lactone
[0066] Add 100 g of oxiracetam acid and 2 g of concentrated sulfuric acid into 200 g of acetone, raise the temperature to 50° C., stir and react for 3 hours, then concentrate under reduced pressure to dryness to obtain oxiracetam acid lactone, and the yield of this step is calculated as 100%. .
[0067] 2. The reaction system for preparing (1R,5R), (1S,5R) and (1R,5S), (1S,5S) type oxiracetam lactone salt
[0068] Add 400 g of acetone and 144 g of D(+)-10-camphorsulfonic acid to the oxiracetam lactone obtained in step 1, raise the temperature to 50° C. and keep stirring for 1 hour, then cool down to 0° C. and grow the crystal for 2 hours.
[0069] At this time, (1R, 5R), (1S, 5R) type oxiracetam acid lactone salt crystallized out in the reaction system, and (1R, 5S), (1S, 5S) type oxiracetam acid lactone salt remained in...
Embodiment 2
[0070] Example 2 The separation of isomers in the reaction system comprising oxiracetam lactone salt isomers
[0071] 1) Take the reaction system of Example 1, filter, wash the filter cake with 40 g of acetone, collect the filter cake, control the temperature at 50° C. and dry under reduced pressure for 6 hours to obtain (1R, 5R), (1S, 5R) type oxiracetam acid lactone salt;
[0072] 3) Collect the filtrate, control the temperature and dry under reduced pressure at 50°C to dryness to obtain (1R, 5S), (1S, 5S) oxiracetam lactone salts.
Embodiment 3
[0073] The preparation of embodiment 3 (R) type oxiracetam
[0074] 1) Add 160 g of methanol to the (1R, 5R) and (1S, 5R) type oxiracetam acid lactone salt obtained in Example 2, lower the temperature to below 10°C, add 2 ml of water, and feed 24 g of ammonia gas to complete The temperature of the ammonia gas was raised to 25°C, and the reaction was carried out under heat preservation and stirring for 15 hours. The sampling was controlled in the center. The purity of the main peak was about 97%, and the remaining raw materials were 2%;
[0075] 2) After the reaction, the temperature was lowered to 0°C and the crystal was incubated for 2 hours, filtered, and the filter cake was washed with 20 g of methanol to obtain the crude product of (R) oxiracetam;
[0076] 3) Add 40g of water and 0.1g of glacial acetic acid and heat up to 60°C to dissolve, filter while hot after dissolving, slowly cool the filtrate to 40-45°C for crystallization for 30 minutes, then cool down to 5°C for 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com