Oxiracetam drug activity composition and preparation method thereof
A technology of drug activity and composition, applied in the field of medicine, can solve the problems of unqualified drug quality control, poor product stability, unstable quality, etc., and achieve the effect of ensuring clinical efficacy, drug safety, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0065] Preparation Example 1: Oxiracetam pharmaceutical active product, prior art product, prepared according to the preparation method disclosed in Japanese Patent JP62026267
[0066] Under the protection of nitrogen, add 11.1Kg of glycinamide hydrochloride, 100L of absolute ethanol and 10.6Kg of anhydrous sodium carbonate into the reaction tank equipped with mechanical stirring, then slowly add 4-chloro-3-hydroxybutyric acid into the reaction solution 16.7Kg of ethyl ester was added in 60 minutes. Then start timing after warming up to reflux, and the reaction time is 24h; after the reaction is completed, filter under reduced pressure while hot, and wash the filter cake with absolute ethanol; take the filtrate, distill the solvent under reduced pressure; evaporate to dryness to obtain a brown viscous liquid, and Dissolve it in 25L of water, pass the above-mentioned aqueous solution through a strong acidic styrene-based cation exchange resin and collect it, then use a strong...
preparation example 2
[0067] Preparation Example 2: Oxiracetam pharmaceutical active product, prior art product, according to the preparation method disclosed in Chinese patent CN101723871
[0068]5h。 Under the protection of nitrogen, add glycinamide hydrochloride 16.6Kg, absolute ethanol 100L and anhydrous sodium carbonate 40Kg to the reaction tank equipped with mechanical stirring, add Tween-20 0.50Kg, react at room temperature for 0. 5h. Next, 23.3 Kg of methyl 4-chloro-3-hydroxybutyrate was added slowly, and the addition was completed in 40 minutes. Then start timing after heating up to reflux, the reaction time is 20h; after 20h of reaction, filter under reduced pressure while hot, and wash the filter cake with absolute ethanol. The filtrate was taken, and the solvent was distilled off under reduced pressure. Evaporate to dryness to obtain a brown viscous liquid, which is recrystallized by adding 25L of absolute ethanol. The solid was precipitated, filtered under reduced pressure, the filt...
Embodiment 1
[0073] Embodiment 1: Oxiracetam pharmaceutically active composition
[0074] Oxiracetam crude product: Oxiracetam crude product prepared according to the method of Preparation Example 1
[0075] A. Dissolution: Dissolve 200g of crude oxiracetam in 600ml of purified water, add 1.0g of activated carbon, heat up to 40°C, control the temperature at 40~50°C, stir and decolorize for 0.5 hours, filter, and collect the filtrate;
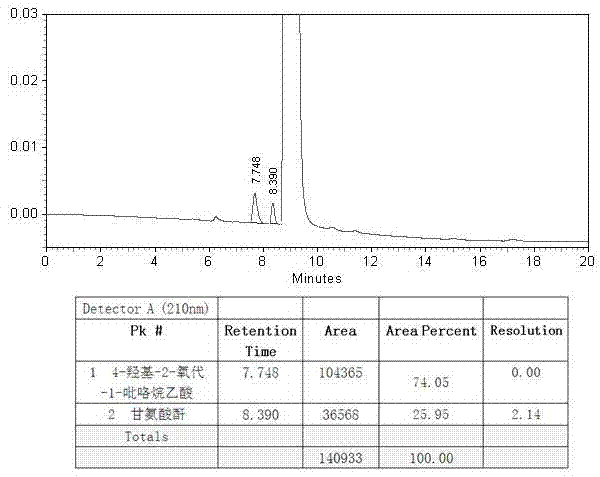
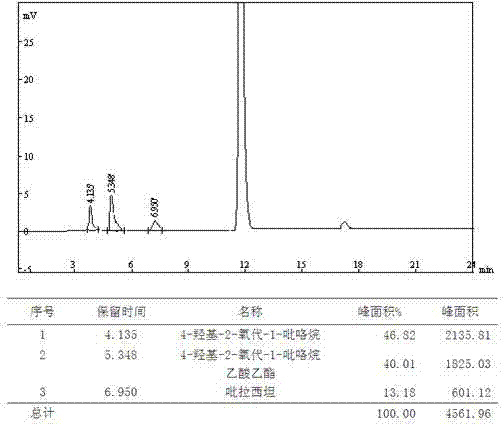
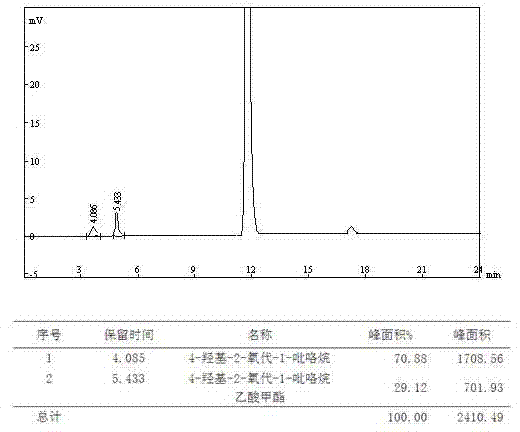
[0076] B. Chromatographic separation: Put the collected filtrate on the chromatographic separation column, use water as the mobile phase, and the stationary phase is silica gel with embedded amino polar groups. The flow rate is 40ml / min, the column temperature is 30°C, and the eluent is collected in sections , combining the eluate with a purity greater than 95%;
[0077] C. Concentration by nanofiltration: Concentrate the eluent by nanofiltration to 1 / 2 of the volume;
[0078] D. Crystallization: Add 15.0L of acetone to the concentrated solution to pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com