Oxazolidinone-based antibacterial drug preparation method
A technology of antibacterial drugs and oxazolidinone, which is applied in the field of medicine, can solve the problems of high raw material cost of catalyst palladium carbon, difficult control of metal palladium residues, and unsuitability for industrial production, and achieve high product quality, easy control of metal residues, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
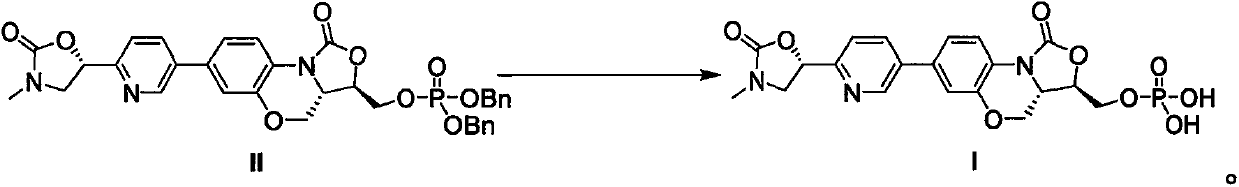
[0034] Weigh phosphate triester (compound of formula II) (10 g) into a reaction flask, add trifluoroacetic acid (100 mL) and stir to dissolve. Cool to -5°C and react at constant temperature for 3 hours, and the reaction is complete as detected by liquid chromatography. Trifluoroacetic acid was distilled off, N,N-dimethylformamide was dissolved, concentrated under reduced pressure until solids were precipitated, and dried to obtain 6.73 g of compound I with a yield of 93% and a liquid phase purity of 99.4%.
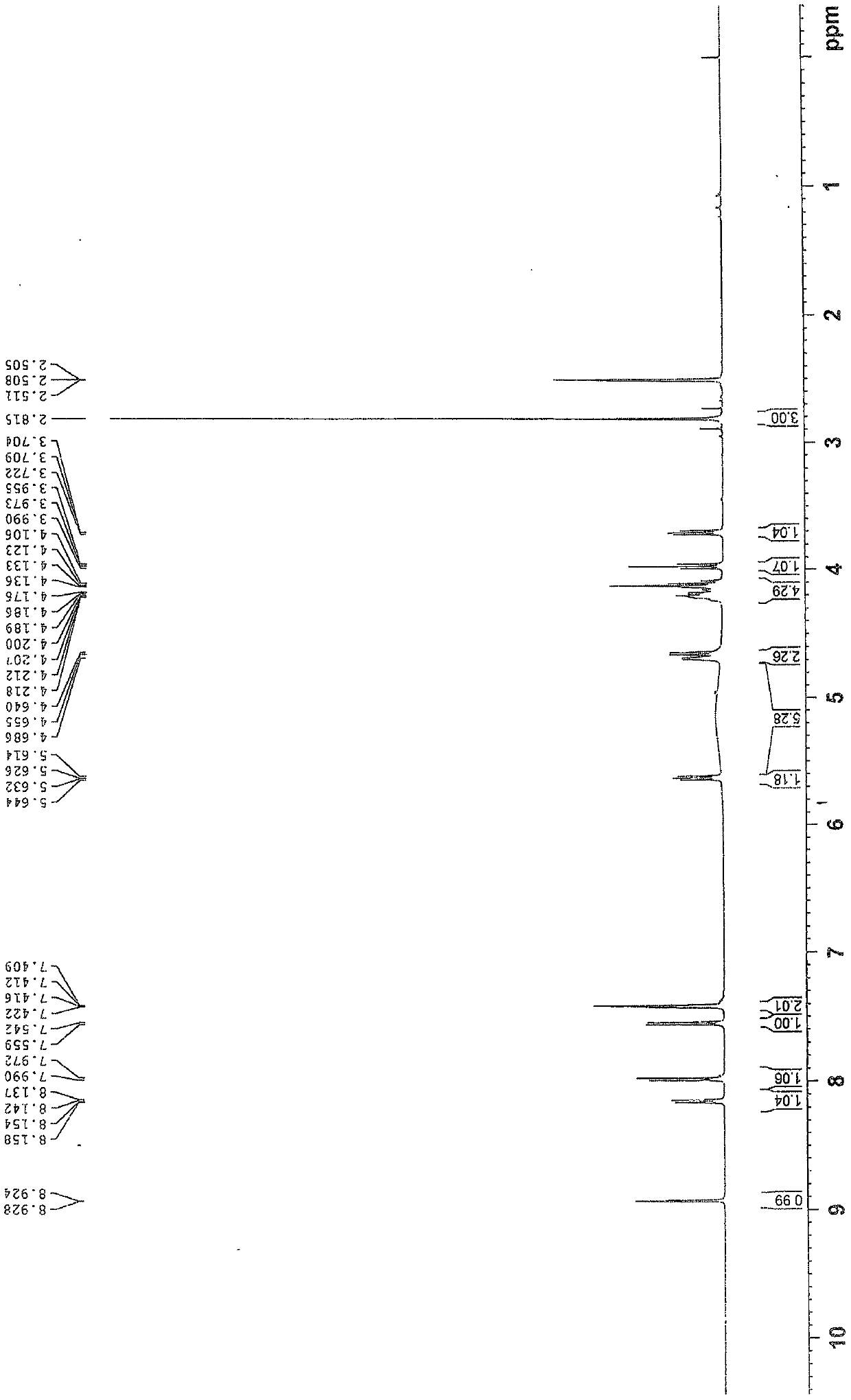
[0035] 1 H NMR ((CD 3 ) 2 SO, 500MHz) δ: 2.82(s, 3H), 3.70~3.72(m, 1H), 3.97(t, J=8.8Hz, 1H), 4.11~4.22(m, 4H), 4.64~4.69(m, 2H ), 5.63(dd, J=9.0, 6.0Hz, 1H), 7.41~7.42(m, 2H), 7.55(d, J=8.4Hz, 1H), 7.98(d, J=9.0Hz, 1H), 8.15 (dd, J=8.4, 2.2Hz, 1H), 8.93(d, J=2.2Hz, 1H);
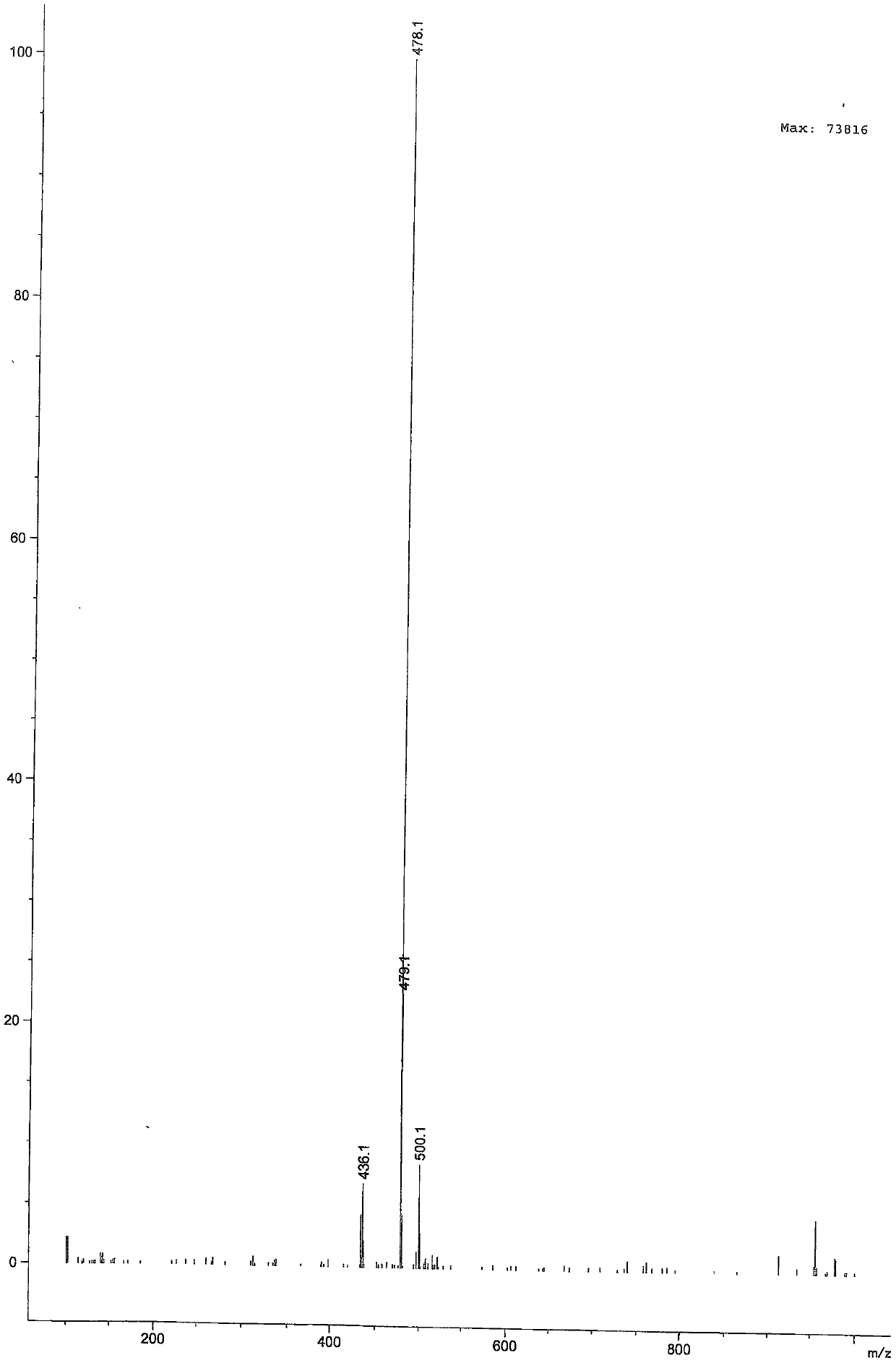
[0036] ESI-MS m / z: 478.1{[M+H] +}.
Embodiment 2
[0038]
[0039] Weigh phosphate triester II (10 g) into a reaction flask, add trifluoroacetic acid (30 mL) and dichloromethane (100 mL) and stir to dissolve. React at room temperature for 2 hours, and the liquid chromatography detects that the reaction is complete. The solvent was evaporated, N, N-dimethylformamide was dissolved, concentrated under reduced pressure until a solid precipitated, and dried to obtain 6.88 g of compound I, with a yield of 95% and a liquid phase purity of 99.7%.
Embodiment 3
[0041]
[0042] Weigh phosphate triester II (10 g) into a reaction flask, add 27% hydrochloric acid isopropanol solution (100 mL) and stir to dissolve. React at room temperature for 2 hours, and the liquid chromatography detects that the reaction is complete. The solvent was evaporated, N, N-dimethylformamide was dissolved, concentrated under reduced pressure until a solid precipitated, and dried to obtain 6.35 g of compound I with a yield of 87% and a liquid phase purity of 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com