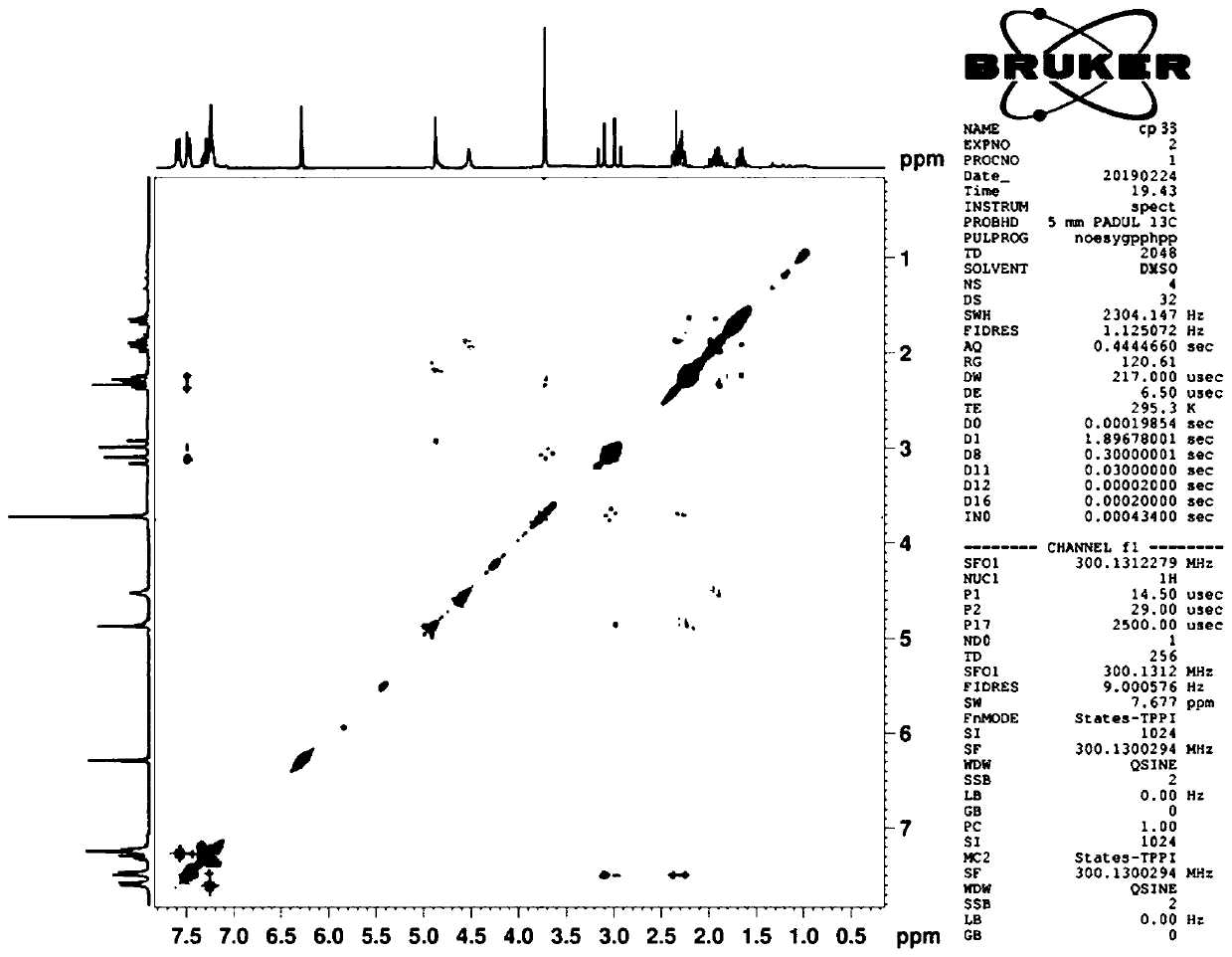
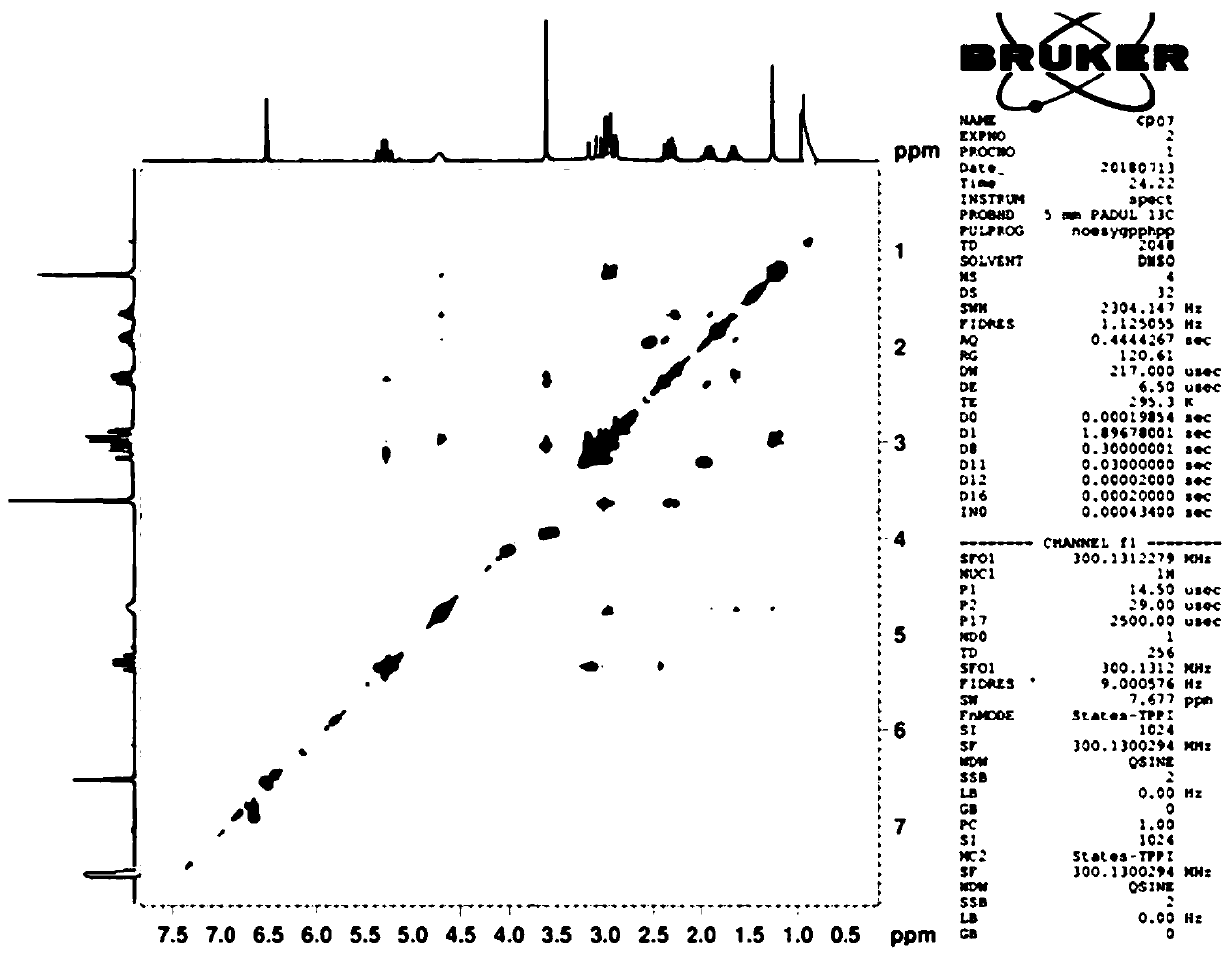
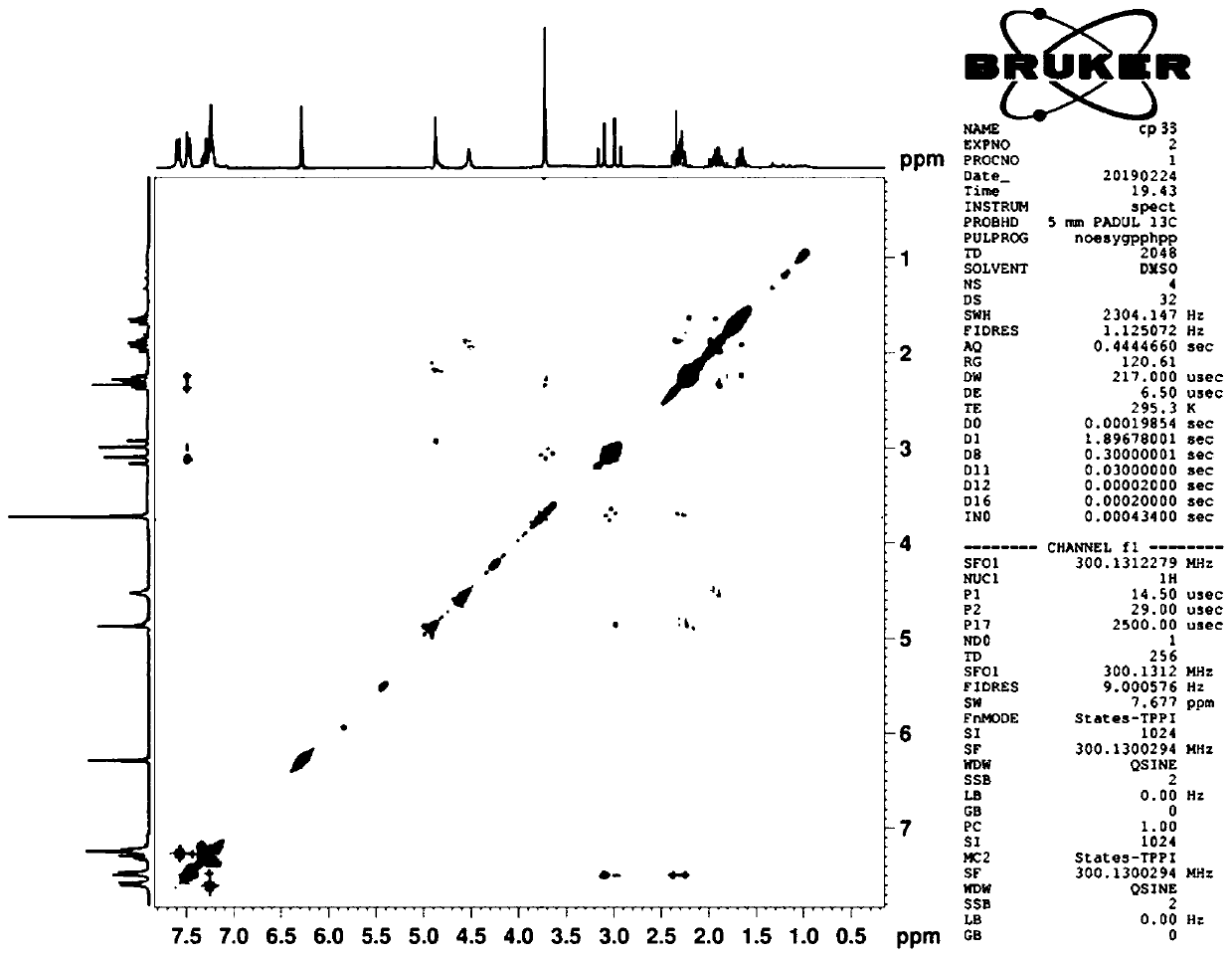
Composition of oxyclopidogrel optical isomer or salt thereof and application of composition
A kind of technology of optical isomer and composition, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0038] This preparation example discloses the preparation method of the compound of formula ⅡA, specifically:
[0039] (S)-2-(2-Chlorophenyl)-2-((S)-7a-hydroxy-2-oxo-2,6,7,7a-tetrahydrothieno[3,2-c]pyridine Synthesis of -5(4H)methyl Acetate
[0040] Step 1. Synthesis of IM1:
[0041]
[0042] At room temperature, slowly drop 35 mL of anhydrous THF suspension with 7.1 g of sodium hydride into 586 mL of anhydrous THF dissolved with 58.6 g of SM1, stir for 30 min, then slowly drop into 70 mL of anhydrous THF with 69.2 g of SM2 dissolved therein and Stir at room temperature for 12 h, and monitor the reaction by TLC iodine display. After the reaction was completed, 650 mL of saturated brine was added and extracted with 1500 mL of DCM. Organic layer with Na 2 SO 4 After drying, the solvent was removed by evaporation under reduced pressure. The residue was purified on a silica gel column to obtain 52.3 g (62.3% yield) of IM1 as a colorless oily liquid (PE / EA=20 / 1 to 5 / 1). L...
preparation example 2
[0059] This preparation example discloses the preparation method of formula IIB, specifically:
[0060] N-Oxo-(S)-2-(2-chlorophenyl)-2-((S)-2-oxo-2,6,7,7a-tetrahydrothiopheno[3,2-c]pyridine Synthesis of -5(4H)yl)methyl acetate.
[0061] Step 1. Resolution of the racemic product
[0062]
[0063] Referring to the patent CN104245707A, after we synthesized the structure of the SMX compound, the obtained racemic product 4.6gSMX was resolved by preparing a chiral column to obtain two relatively pure enantiomers SMX-1 and SMX-2. SMX-1 is 1.24g and SMX-2 is 1.51g. (Yield 59.8%) LC-MS (ESI) [M+H + ] + =338.8(M+H + ) is consistent with the structure.
[0064] Step 2. Synthesis of formula IIB:
[0065]
[0066] Put 1.24g of SMX-1 and 10mL of glacial acetic acid into a 50mL three-neck flask at room temperature, add 1ml of hydrogen peroxide dropwise in an ice bath, raise the temperature to 80°C for 2 hours after the addition, and monitor the reaction by TLC (developer: ethyl ...
Embodiment 1
[0068] This example discloses a composition comprising 4% of the compound of formula IIA.
[0069] The optical isomer (7aS,2'S)-2-oxo-clopidogrel synthesized according to CN104245707A has a purity of 98.0% (nitrogen protection, no impurity compound of formula IIA or formula IIB). To 0.96g (7aS,2'S)-2-oxo-clopidogrel, add 0.04g of the compound of formula IIA with a purity of 98.0%, and mix evenly to obtain a composition with a content of compound of formula IIA of 4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com