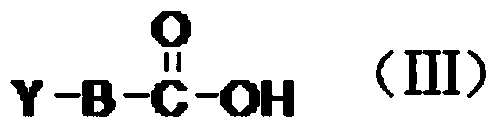Preparation of benzamide histone deacetylase inhibitor with differentiation and anti-proliferation activity
A deacetylase and benzamide technology, applied in organic chemistry, drug combination, anti-tumor drugs, etc., can solve problems such as nonconformity, rapid metabolism, increase production cost, etc., and achieve mild reaction conditions and simple purification methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
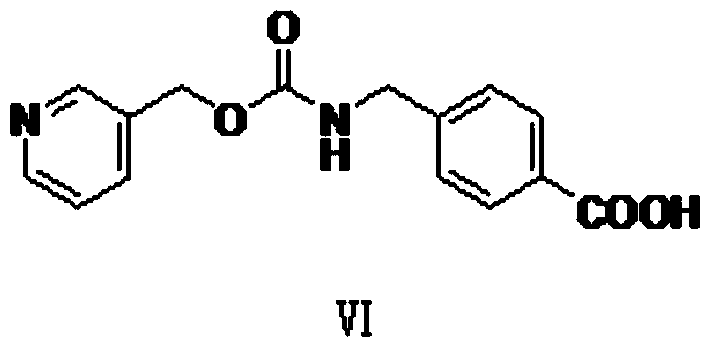
[0056] The synthesis of embodiment 1 compound VI
[0057]
[0058] Add N,N to the 500ml reaction bottle , -Carbonyldiimidazole 75g (462.5mmol), tetrahydrofuran 100ml, stir well, at room temperature (10 ~ 20 ℃), add dropwise 50ml of tetrahydrofuran solution of 3-pyridinemethanol (25.2g, 231mmol) that has been prepared, after the dropwise addition React at room temperature (10-20°C) for 6h. Cool the system down to about 0°C, add 34.9g (231mmol) of 4-aminomethylbenzoic acid under temperature control at about 0°C, stir for 30min, then add 59.7 g of N,N-diisopropylethylamine dropwise at about 0°C g (462.5mmol), react at 0-10°C for 8h after the dropwise addition. After the reaction, control the temperature at 0-20°C and add concentrated hydrochloric acid dropwise to adjust the pH of the system to 4-5. After the adjustment, stir at 0-20°C for 4 hours. Filter, wash the filter cake twice with 500ml of purified water, and dry the wet product at 40-50°C for 8 hours. Obtained light...
Embodiment 2
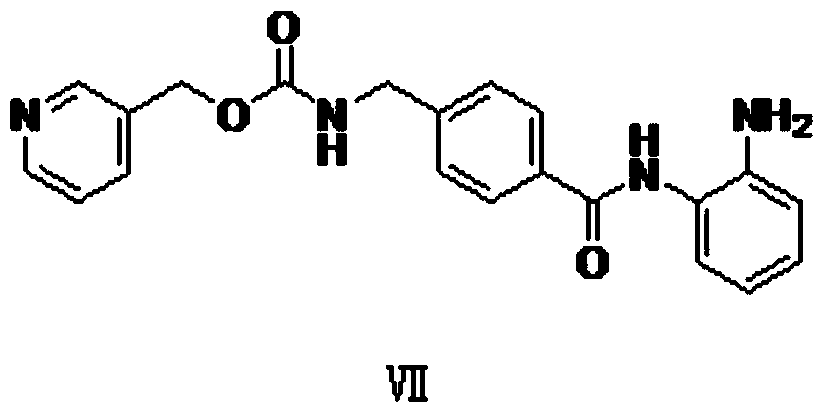
[0061] Synthesis of Example 2 Compound VII
[0062]
[0063] Put 42g (146.7mmol) of compound VI and 100ml of dimethylformamide into a 500ml reaction flask, add 35.7g (220mmol) of N,N,-carbonyldiimidazole at room temperature (10-20°C), ~20°C) and stirred for 2h. Cool the system down to about 0°C, control the temperature at about 0°C, add dropwise 150ml of a dimethylformamide solution of o-phenylenediamine (47.6g, 440.1mmol) that has been prepared, and stir for 30min at about 0°C after the drop is complete. Control the temperature at about 0°C and add 28.2g (293.4mmol) of methanesulfonic acid dropwise, and react at 0-20°C for 8h after the drop is completed. After the reaction, the reaction liquid was poured into 2500ml of purified water, and was beaten at room temperature (10-20° C.) for 12 hours. After filtering, the filter cake was washed twice with 800ml of purified water, and after being sucked dry, the wet product was vacuum-dried at 40-50°C for 20 hours to obtain ligh...
Embodiment 3
[0068] The synthesis of embodiment 3 compound VI
[0069] Add 37.5g (231mmol) of N,N,-carbonyldiimidazole and 200ml of tetrahydrofuran into a 500ml reaction flask, stir evenly, and add 3-pyridinemethanol (25.2g, 231mmol) dropwise at room temperature (10-20°C) ) in 60ml of tetrahydrofuran solution, react at room temperature (10-20° C.) for 6 hours after the dropwise addition. Cool the system down to about 0°C, add 69.8g (462mmol) of 4-aminomethylbenzoic acid at about 0°C, stir for 30min, add 35.1g (346.5mmol) of triethylamine dropwise at about 0°C, drop After the addition, react at 0-10°C for 8h. After the reaction, control the temperature at 0-20°C and add concentrated hydrochloric acid dropwise to adjust the pH of the system to 4-5. After the adjustment, stir at 0-20°C for 4 hours. After filtering, the filter cake was washed twice with 500ml of purified water, and after being sucked dry, the wet product was air-dried at 40-50°C for 8 hours. The light yellow solid compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com