Method for preparing cariprazine and intermediate thereof
A cariprazine and intermediate technology, which is applied in the field of pharmaceutical compound preparation, can solve the problems of low industrialized production efficiency, unsuitable for industrialized production, harsh synthesis conditions, etc., and achieves the advantages of long reaction time, low equipment requirements and simplified post-processing. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
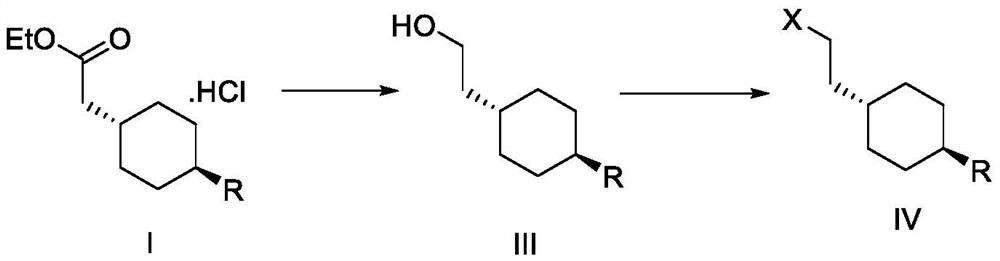
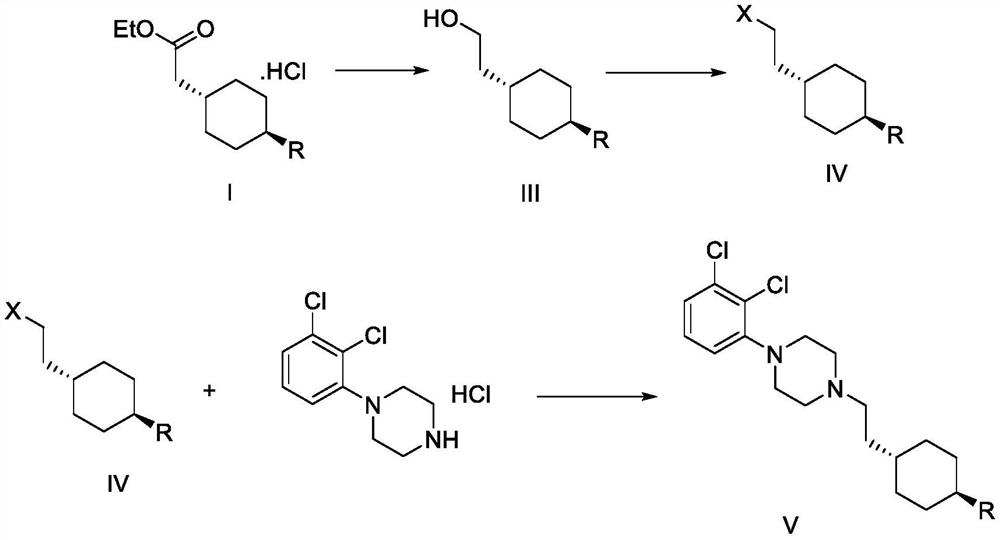
[0103] Preparation of trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-ethyl acetate
[0104] In a 250ml three-necked flask, add 105g of dichloromethane, 15g (67.65mmol) trans-4-aminocyclohexane acetate hydrochloride, 7.8g (77.08mmol) triethylamine, stir and cool down to 5-10 °C, a solution consisting of 17.3 g (79.27 mmol) of di-tert-butyl dicarbonate and 45 g of dichloromethane was added dropwise thereto, and the temperature was controlled at 5-10 °C during the dropwise addition. After the dropwise addition was completed, the temperature was raised to 20-30° C., and the mixture was kept stirring for 2 hours. After HPLC detection, the reaction of the raw materials was complete (trans-4-aminocyclohexane acetate hydrochloride was not detected). Add 40g of 5% sodium carbonate dropwise thereto, stir for 15min after addition, separate layers, wash the organic layer with 15g of water, dry the organic layer with 10g of anhydrous sodium sulfate, filter with suction, concentra...
Embodiment 2
[0106] Preparation of trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}ethanol
[0107] In a 500ml three-necked flask, add 17.28g (0.06mol) trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-ethyl acetate prepared in Example 1, 104g tetrahydrofuran, 5.04g (0.13mol) sodium borohydride; temperature control at 18-25°C, dropwise add a solution consisting of 8.07g (0.06mol) aluminum chloride and 60g tetrahydrofuran, dropwise, keep stirring for 3 hours, HPLC detection showed that the reaction of raw materials was complete (trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-ethyl acetate was not detected). After the reaction is complete, transfer the reaction solution to a 1L three-necked flask, lower the temperature of the system to 5-10°C, add 172g of toluene to dilute the reaction solution, control the temperature at 5-10°C, and slowly add 207g of water dropwise to it. About 12g concentrated hydrochloric acid to adjust the pH of the system to 2-4. After the adj...
Embodiment 3
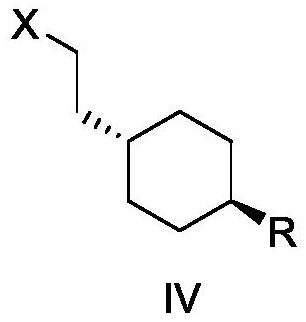
[0109] Preparation of trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}bromoethane
[0110] In a 250ml three-necked flask, add 6g (24.65mmol) trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}ethanol, 120g dichloromethane, stir to dissolve, Lower the temperature of the system to 0-5° C., and add 7.8 g (29.75 mmol) of triphenylphosphine and 9.8 g (29.55 mmol) of carbon tetrabromide to it in sequence. After the addition, stir at 0-5°C for 1h, then raise the temperature to 20-30°C and stir for 1h, perform HPLC detection, the raw material reaction is complete (trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino ]-cyclohexyl}ethanol was not detected). The reaction solution was concentrated and dried, then separated on a silica gel column, and eluted with n-heptane-ethyl acetate (volume ratio 5:1) to obtain 7.4 g of a white solid, which was the compound trans 2-{1-[4-(N- tert-butoxycarbonyl)-amino]-cyclohexyl}bromoethane, the purity is 100% (ELSD detector detection), and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com