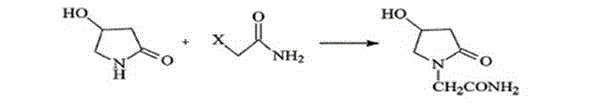
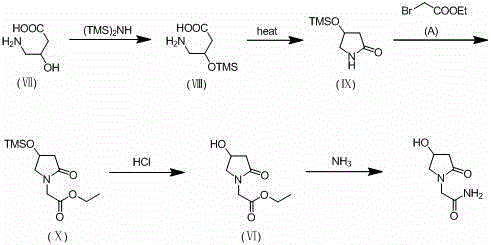
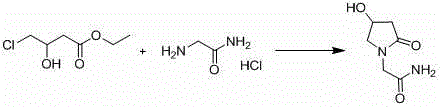
Synthesis method of oxiracetam
A synthesis method and compound technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of many by-products, harsh reaction conditions, low product and yield, etc., to improve purity and yield, suitable for industrial production, Avoid by-product effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] R 1 tert-butyldimethylsilyl
[0037] Preparation of 4-tert-butyldimethylsiloxy-2-oxopyrrolidone
[0038] Add 101.1 g (1.0 mol) of 4-hydroxy-2-oxopyrrolidone to 500 ml of DMF to dissolve, add 165.8 g (1.1 mol) of TBDMSCl and 102.1 g (1.5 mol) of imidazole, and react for 18 hours. The reaction solution was added to 1500.0g water, extracted with methyl tert-butyl ether (1000ml×3), washed once with 1000ml water, dried over anhydrous sodium sulfate, filtered and concentrated to dryness, and the residue was crystallized from n-heptane, filtered and dried to obtain 4 - tert-butyldimethylsiloxy-2-oxopyrrolidone.
Embodiment 2
[0040] R 1 tert-butyldiphenylsilyl
[0041] Preparation of 4-tert-butyldiphenylsilyl-2-oxopyrrolidone
[0042] Add 200g (1.0mol) of 4-hydroxy-2-oxopyrrolidone into 500ml of DMF to dissolve, add 300g (1.1mol) of tert-butyldiphenylchlorosilane and 190.5g (1.5mol) of imidazole, and keep it at 40°C for 10 hours . The reaction solution was added to 2000.0g water, extracted with methyl tert-butyl ether (1500ml×3), washed once with 1000ml water, dried over anhydrous sodium sulfate, filtered and concentrated to dryness, and the residue was crystallized from n-heptane, filtered and dried to obtain 4 - tert-butyldiphenylsilyl-2-oxopyrrolidone.
Embodiment 3
[0044] Preparation of Oxiracetam
[0045] Take 215.36 g of 4-tert-butyldimethylsilyloxy-2-oxopyrrolidone and 200.4 g of ethyl 2-bromoacetate in Example 1 and dissolve them in 800 ml of THF, and slowly add 1.6 M HMDSLi dropwise at 5°C Tetrahydrofuran solution 750ml, after dropping, keep the temperature and stir for 1 hour. TLC detection (developing agent dichloromethane: methanol = 30:1, phosphomolybdic acid for color development) the reaction is complete, add 900ml of water, separate the water layer, concentrate the organic layer to dryness, add 900ml of ethyl acetate to the residue, and wash once with water . Concentrate the ethyl acetate layer to dryness, add 600ml of n-heptane to crystallize, wash with 100ml of n-heptane, and dry to obtain ethyl 2-(4-tert-butyldimethylsilyloxy-2-oxopyrrolidin-1-yl)acetate Ester, the yield is 85%, and the HPLC detection shows that the purity is greater than 98%.
[0046] Take 301.45 g of the above-mentioned product 2-(4-tert-butyldimethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com