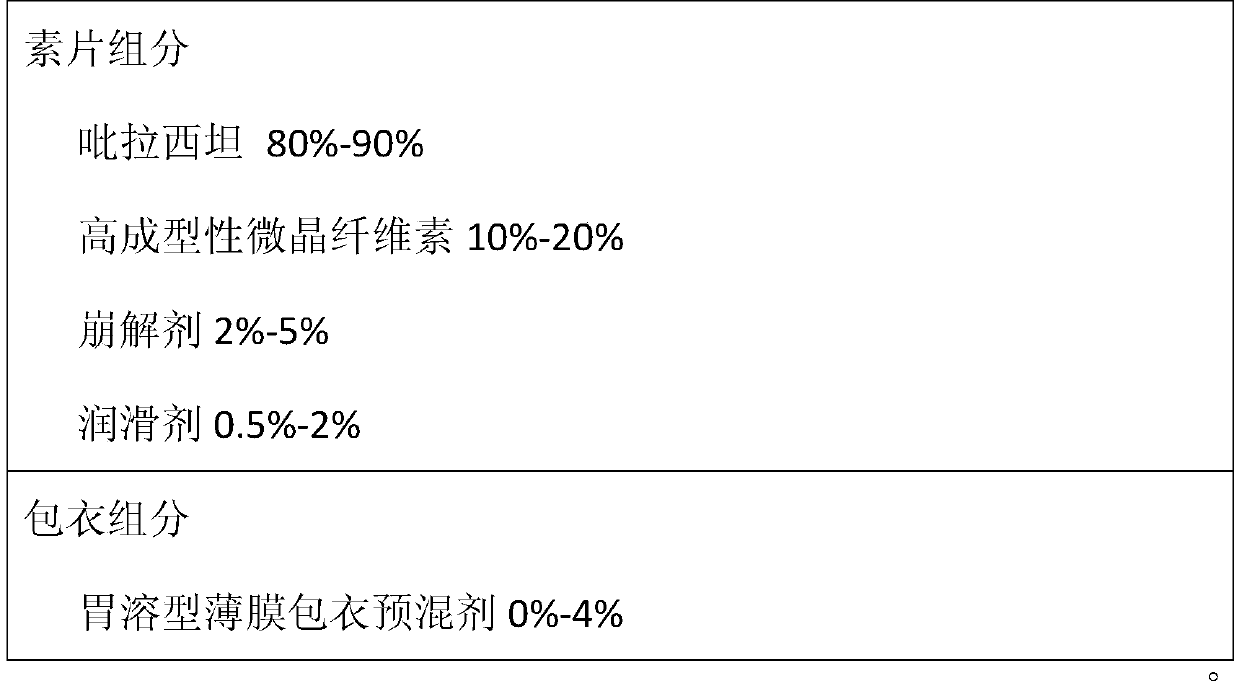
Piracetam tablet composition with high drug loading capacity and preparation method thereof
A technology of piracetam tablet and high drug loading, which is applied in the field of pharmaceutical composition and its preparation, which can solve the problems of inconvenient use, difficulty in swallowing, and occurrence of fragments for patients, so as to ensure effectiveness and safety, The operation process is simple and smooth, and the size is suitable for the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
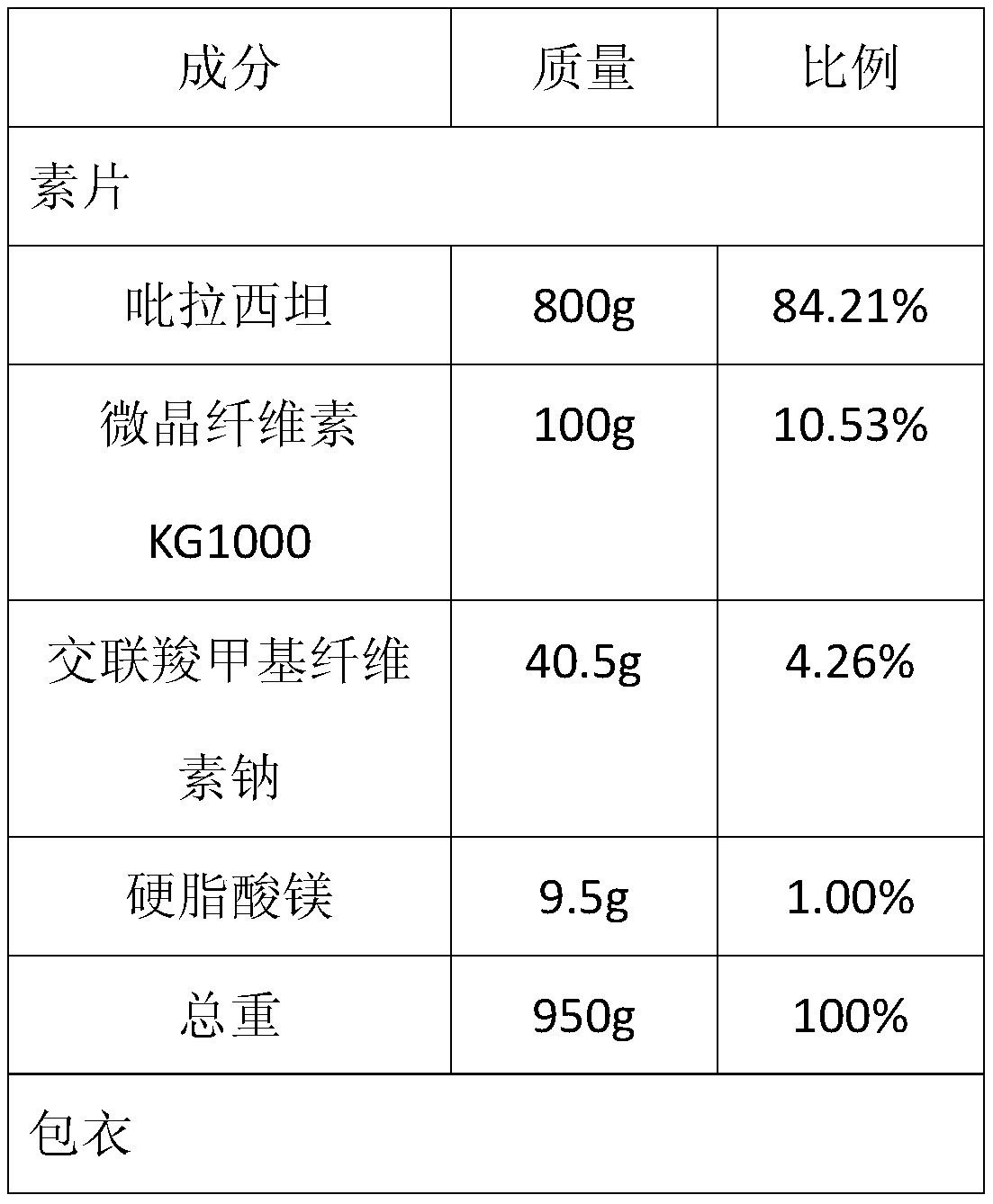
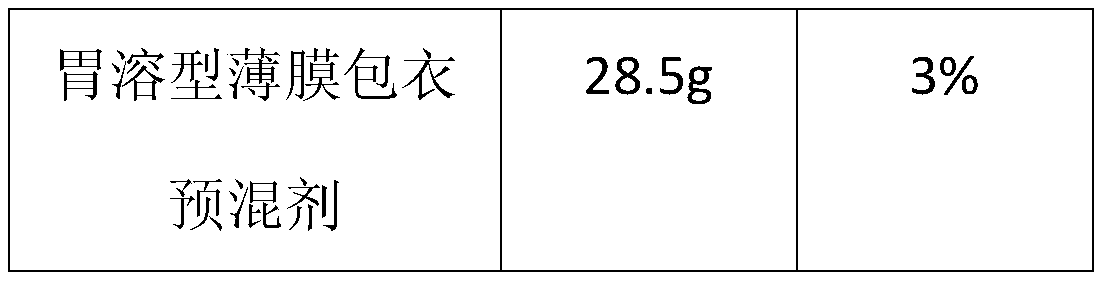
[0034] Example 1—prescription of high-drug-loaded piracetam tablets (specification: 0.8 g) and its preparation method (based on 1000 tablets).
[0035]
[0036]
[0037] Preparation:
[0038] 1. Pass the piracetam raw material and magnesium stearate through a 40-mesh sieve to granulate;
[0039] 2. Add 800g of granulated piracetam API, 100g of highly moldable microcrystalline cellulose KG1000 and 40.5g of croscarmellose sodium into the hopper mixer according to the prescription ratio, and set the mixing speed to 10 rpm / minutes, start the machine and mix for 20 minutes, then add 9.5g magnesium stearate, set the mixing speed to 10 rpm, and mix for 5 minutes;
[0040] 3. Add the intermediate material obtained after mixing into the tablet press for tableting, control the tableting speed to 80,000 tablets / hour, and control the tablet hardness to 90-130N;
[0041] 4. Put the plain tablets into a high-efficiency coating machine, control the temperature of the tablet bed at 3...
Embodiment 2
[0042] Example 2—prescription of high-drug-loaded piracetam tablets (specification: 1.2 g) and its preparation method (calculated as 1000 tablets).
[0043]
[0044]
[0045] Preparation:
[0046] 1. Pass the piracetam bulk drug, colloidal silicon dioxide, and magnesium stearate through a 40-mesh sieve to granulate;
[0047] 2. According to the prescription ratio, add 1200g of piracetam bulk drug, 75g of high-formability microcrystalline cellulose KG802, 150g of high-formability microcrystalline cellulose UF711 and 45g of croscarmellose sodium into the hopper for mixing In the machine, set the mixing speed at 15 rpm, start the machine and mix for 25 minutes, then add 15 g of colloidal silicon dioxide and 15 g of magnesium stearate, set the mixing speed at 10 rpm, and mix for 5 minutes;
[0048] 3. Put the intermediate material obtained after mixing into the tablet machine for tableting, control the tableting speed to 100,000 tablets / hour, and control the tablet hardness...
Embodiment 1-4
[0063] Example 1-4 High drug-loaded piracetam tablet friability test
[0064] Equipment: FT-2000 friability tester
[0065] Test product: the uncoated tablet prepared in Example 1, Example 2, Comparative Example 1-2 Test method: the weight of each test product tablet is >0.65g, and 10 tablets are taken. Use a hair dryer to blow off the falling powder, weigh it accurately, put it in a cylinder, and rotate it 100 times. Take it out, remove the powder in the same way, weigh it precisely, and calculate the weight loss. ("Chinese Pharmacopoeia" 2015 Edition Four General Rules 0923).
[0066] Results and Discussion: The information summary and friability test results of each test product are shown in the table below.
[0067]
[0068]
[0069] As can be seen from the test results, the piracetam tablet brittleness prepared by the high drug-loaded piracetam tablet composition provided by the invention and its preparation method is less than 0.1-0.3 (far lower than the Chinese...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com