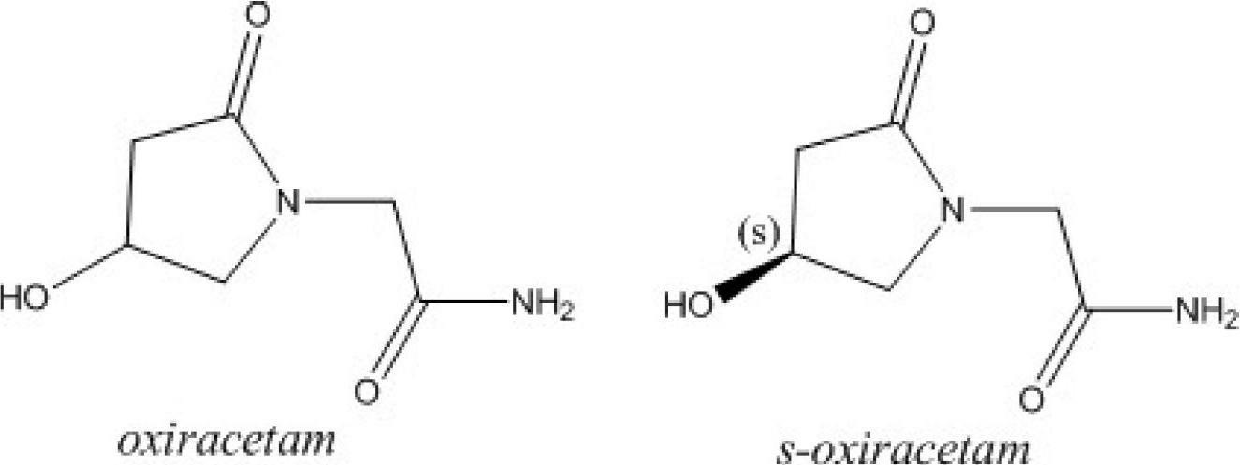
Freeze-dried powder injection of L-oxiracetam and process for preparing freeze-dried powder injection
A freeze-dried powder for injection and freeze-drying technology is applied in the field of freeze-dried powder injection of levo-oxiracetam and its preparation technology, which can solve the problems of decreased solubility, high solution viscosity, long freeze-drying time and the like, and achieves improved solubility, Increased stability and rapid reconstitution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
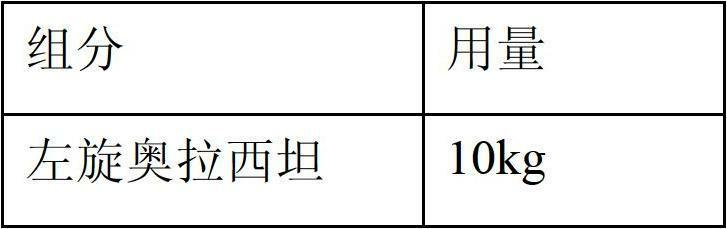
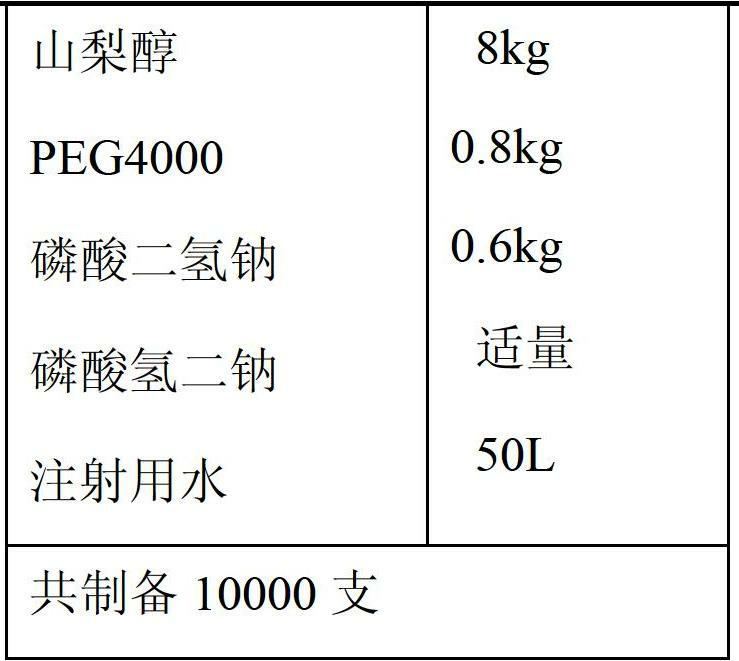
[0031] Embodiment 1 prescription 1 and preparation technology
[0032] Prescription 1:
[0033]
[0034]
[0035] Preparation Process:
[0036] 1) Mix 10Kg levoxiracetam and 0.8Kg PEG4000 evenly and disperse them in 35L of hot water at 45-50°C. After the dissolution is complete, add 8kg sorbitol and 0.6kg sodium dihydrogen phosphate to dissolve completely.
[0037] 2) Adjust the pH of the solution to 4.0 with 0.2mol / L disodium hydrogen phosphate solution,
[0038] 3) Add water to full volume, stir for 10 minutes,
[0039] 4) Decarburize 0.1% w / v activated carbon for 15 minutes, filter through a 1 μm titanium rod filter to remove carbon, and filter through a 0.45 μm cartridge filter;
[0040] 5) Sampling after filtration with a 0.22 μm terminal filter for intermediate inspection;
[0041] 6) After passing the inspection, it is potted and sealed in a vial;
[0042] 7) Freeze-drying:
[0043] First put the subpackaged medicines on the inner partition of the freeze-dry...
Embodiment 2
[0046] Embodiment 2 prescription 2 and preparation technology
[0047] Prescription 2:
[0048]
[0049]
[0050] Preparation Process:
[0051] 1) Mix 10kg of levoxiracetam and 0.6kg of PEG6000 evenly and disperse in 40L of hot water at 45-50℃. After the dissolution is complete, add 8k of mannitol and 0.4kg of sodium dihydrogen phosphate to dissolve completely.
[0052] 2) Adjust the pH of the solution to 5.0 with 0.2mol / L disodium hydrogen phosphate solution,
[0053] 3) Add water to full volume, stir for 10 minutes,
[0054] 4) Decarburize 0.1% w / v activated carbon for 15 minutes, filter through a 1 μm titanium rod filter to remove carbon, and filter through a 0.45 μm cartridge filter;
[0055] 5) Sampling after filtration with a 0.22 μm terminal filter for intermediate inspection;
[0056] 6) After passing the inspection, it is potted and sealed in a vial;
[0057] 7) Freeze-drying:
[0058] First put the subpackaged medicines on the inner partition of the freeze-...
Embodiment 3
[0061] Embodiment 3 prescription 3 and preparation technology
[0062] Prescription 3:
[0063]
[0064] Preparation Process:
[0065] 1) Mix 10kg of levoxiracetam and 0.8kg of PEG6000 evenly and disperse in 40L of hot water at 45-50°C. After the dissolution is complete, add 10kg of lactose and 0.5kg of sodium dihydrogen phosphate to dissolve completely.
[0066] 2) Adjust the pH of the solution to 5.5 with 0.2mol / L disodium hydrogen phosphate solution,
[0067] 3) Add water to full volume, stir for 10 minutes,
[0068] 4) Decarburize 0.1% w / v activated carbon for 15 minutes, filter through a 1 μm titanium rod filter to remove carbon, and filter through a 0.45 μm cartridge filter;
[0069] 5) Sampling after filtration with a 0.22 μm terminal filter for intermediate inspection;
[0070] 6) After passing the inspection, it is potted and sealed in a vial;
[0071] 7) Freeze-drying:
[0072] First put the subpackaged medicines on the inner partition of the freeze-drying b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com