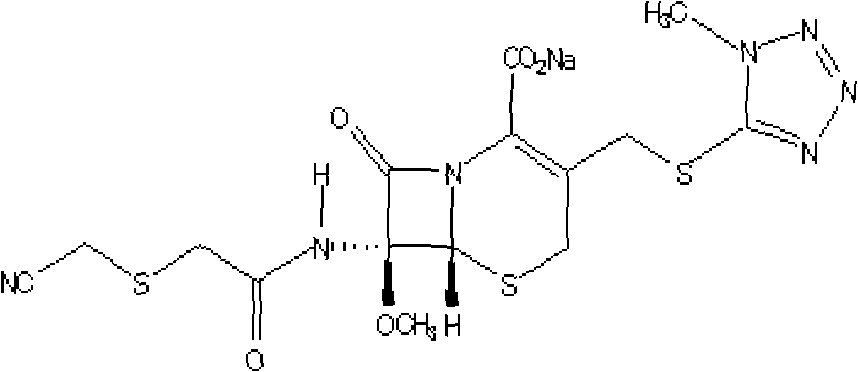
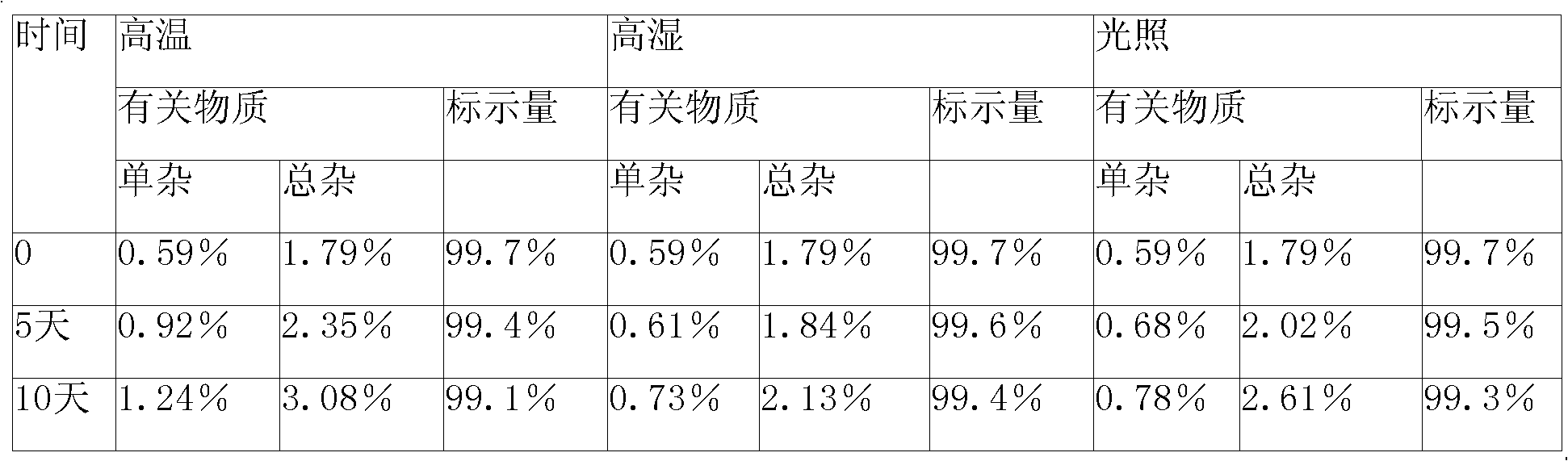
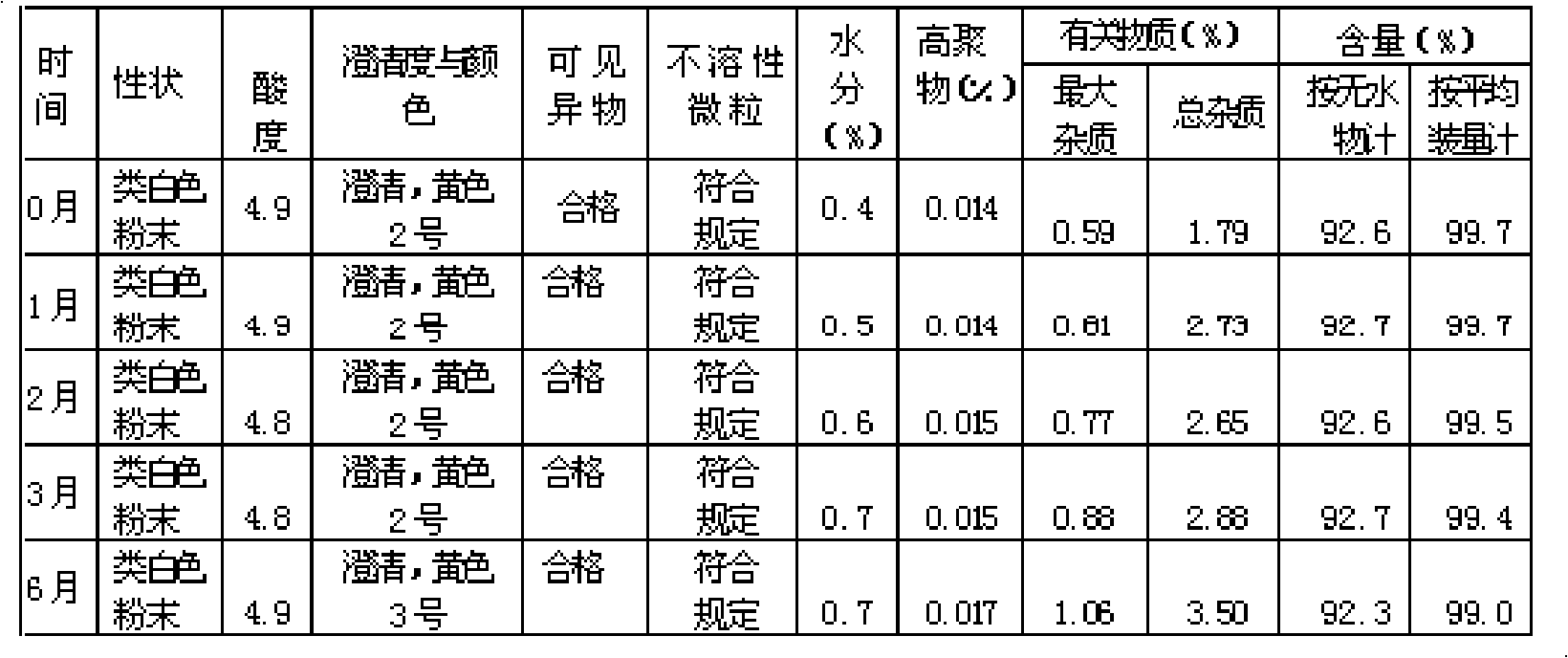
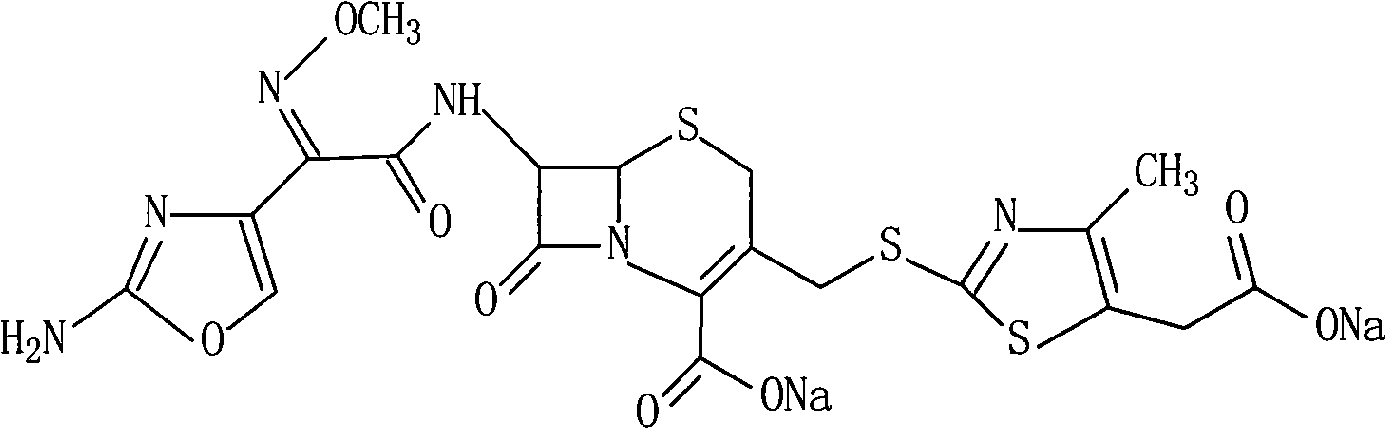
Patents
Literature
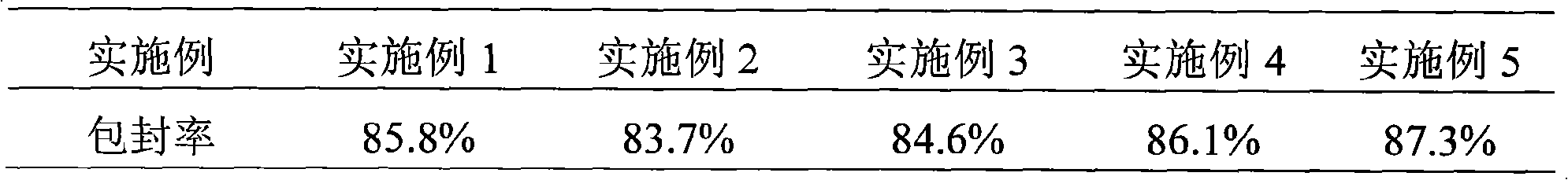
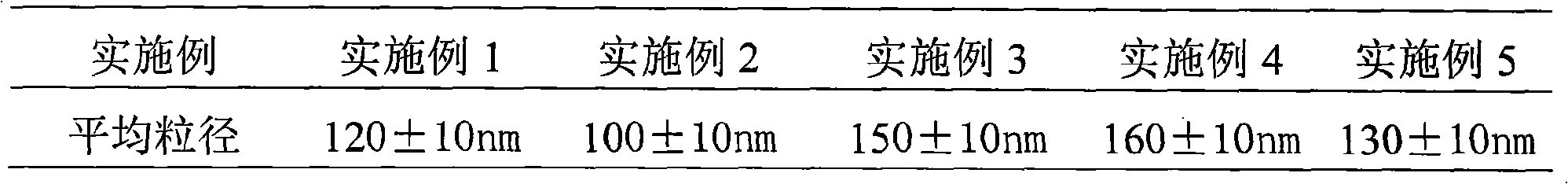
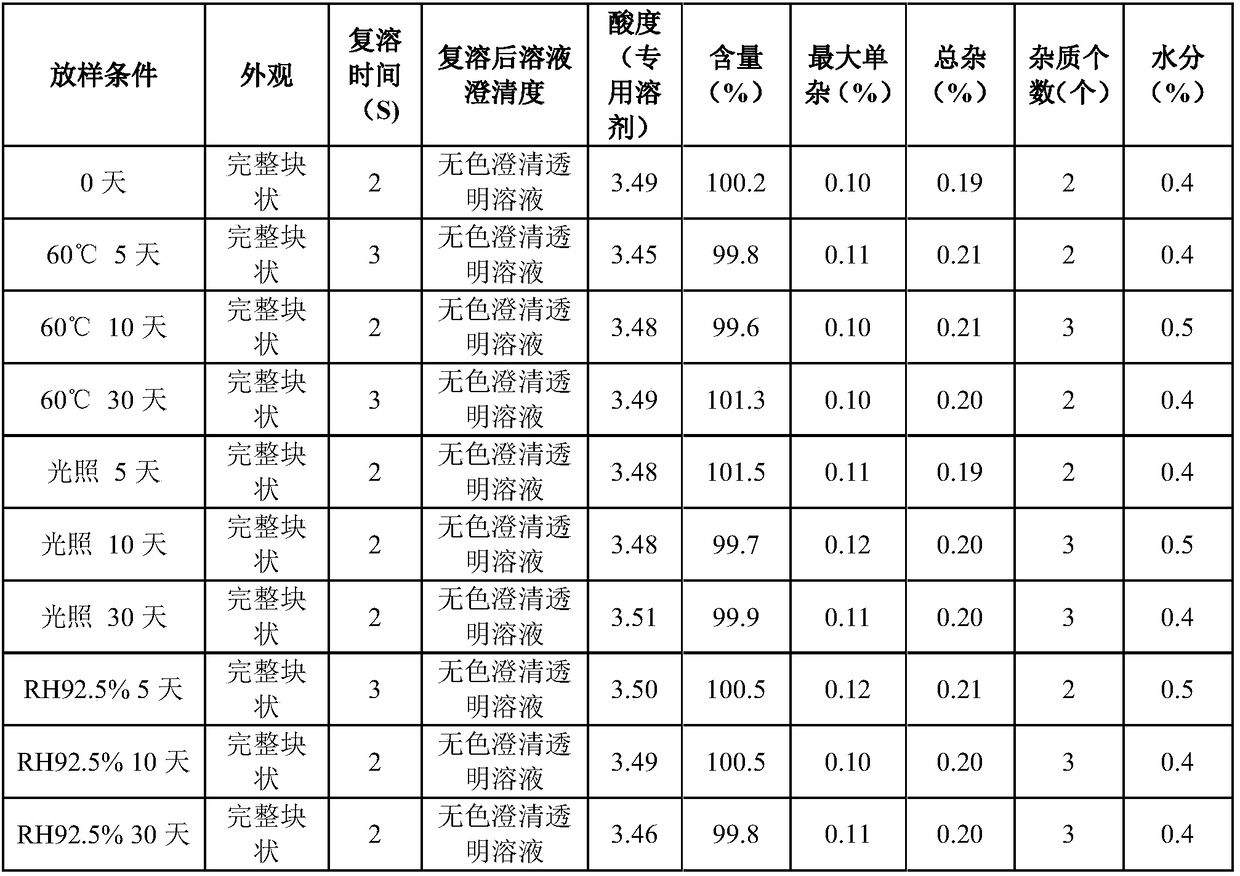
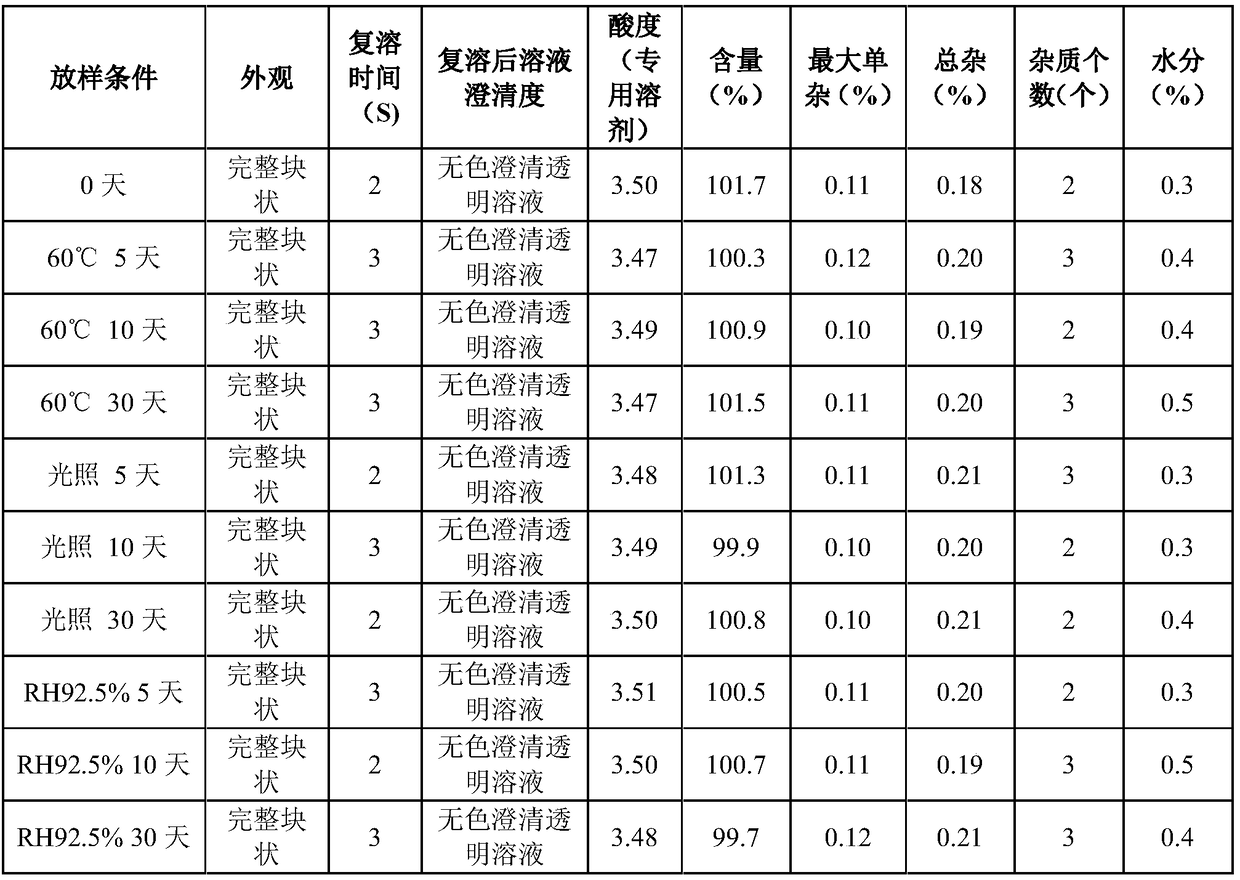
80results about How to "Rapid reconstitution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
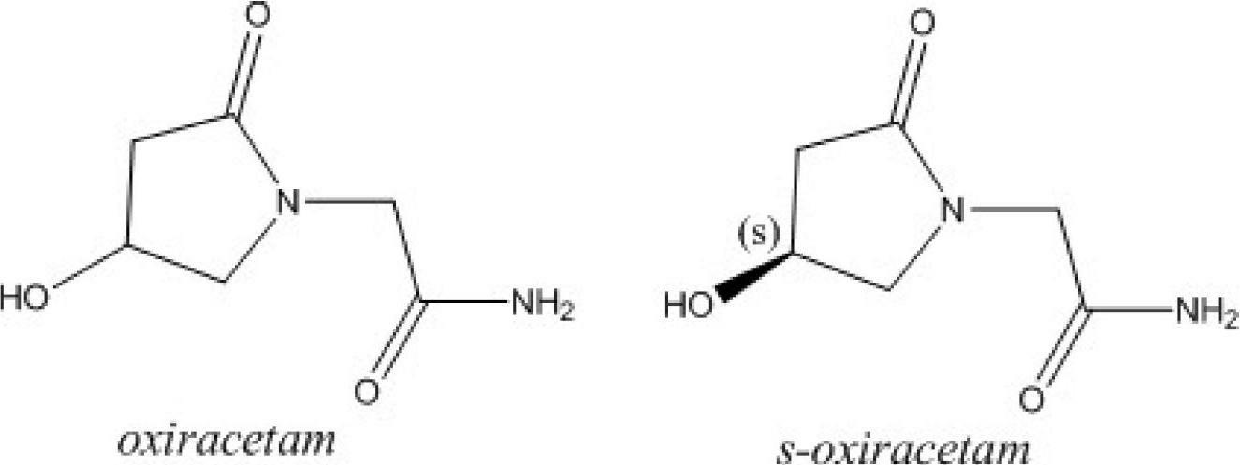
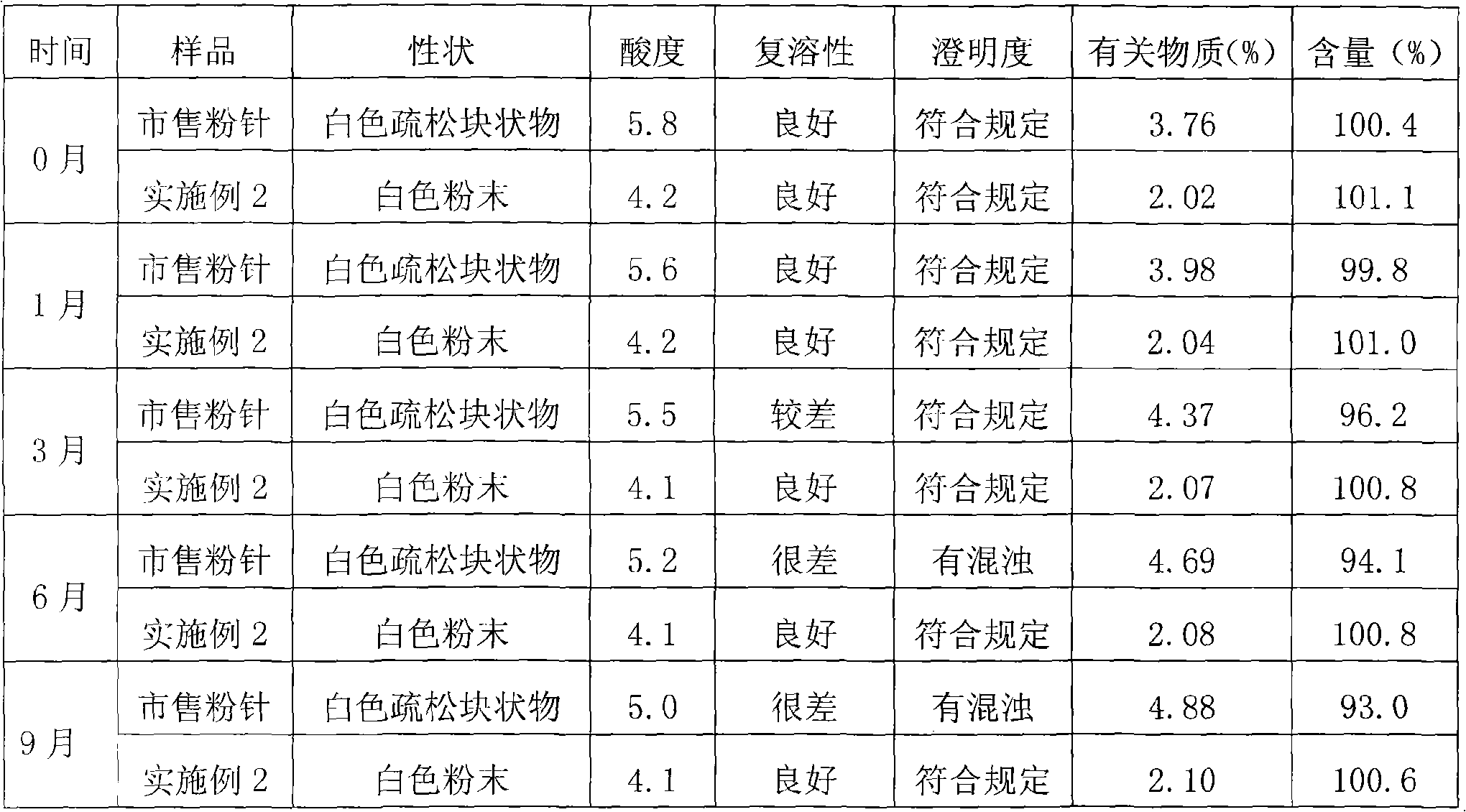
Freeze-dried powder injection of L-oxiracetam and process for preparing freeze-dried powder injection
ActiveCN102670527AFast sublimationShorten freeze-drying timePowder deliveryOrganic active ingredientsPolyethylene glycolEngineering
The invention relates to a freeze-dried powder injection of L-oxiracetam and a process for preparing the freeze-dried powder injection. The freeze-dried powder injection comprises, by weight, 1 part of the L-oxiracetam, 0.1 to 10 parts of an excipient, 0.01 to 1 part of an antisticking agent and 0.01 to 0.5 part of a pH regulator, wherein polyethylene glycol (PEG) series of products serve as the antisticking agent preferably. The freeze-dried powder injection which is prepared on the basis of a prescription according to the process has the advantages of being short in freeze-drying time, attractive in appearance, short in redissolving time and high in stability.
Owner:南京博德生物制药有限公司
Method for preparing powder injection using superfine communication technique and prepared products
InactiveCN101332188AReduce contentUniform colorAntibacterial agentsOrganic active ingredientsLatamoxefClindamycin Phosphate
The present invention relates to a method of using a superfine pulverizing technology to prepare sterile powder for injection (powder injection) of chemical medicine and the prepared medicine powder injection. Invert sugar, clindamycin phosphate, cefpiramide sodium, cefepime hydrochloride, latamoxef sodium or cefmetazole sodium are preferable as the chemical medicine.
Owner:HAINAN LINGKANG PHARMA CO LTD
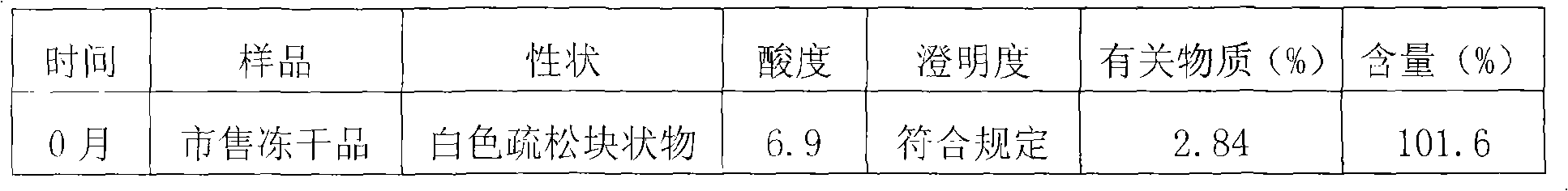
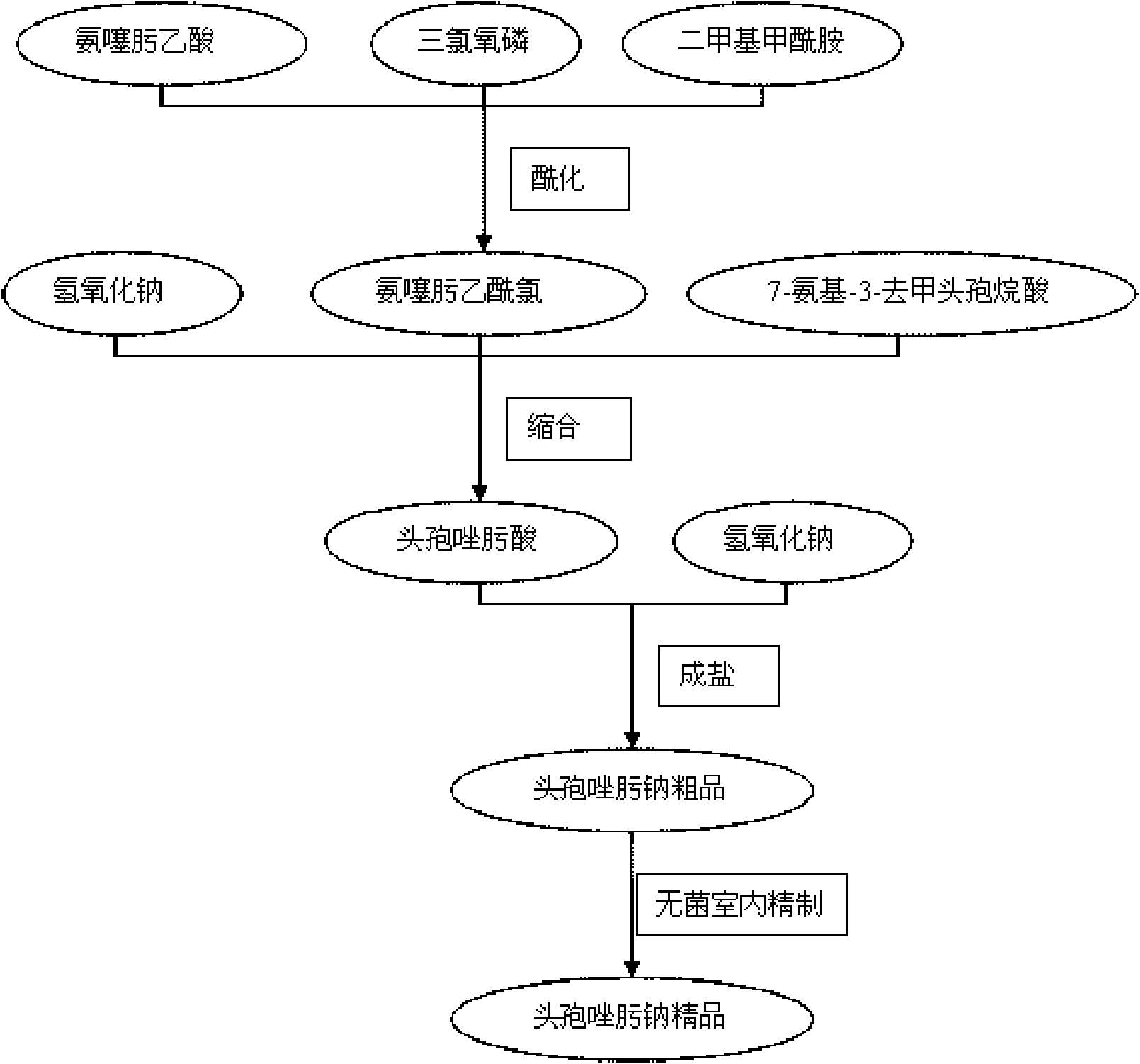
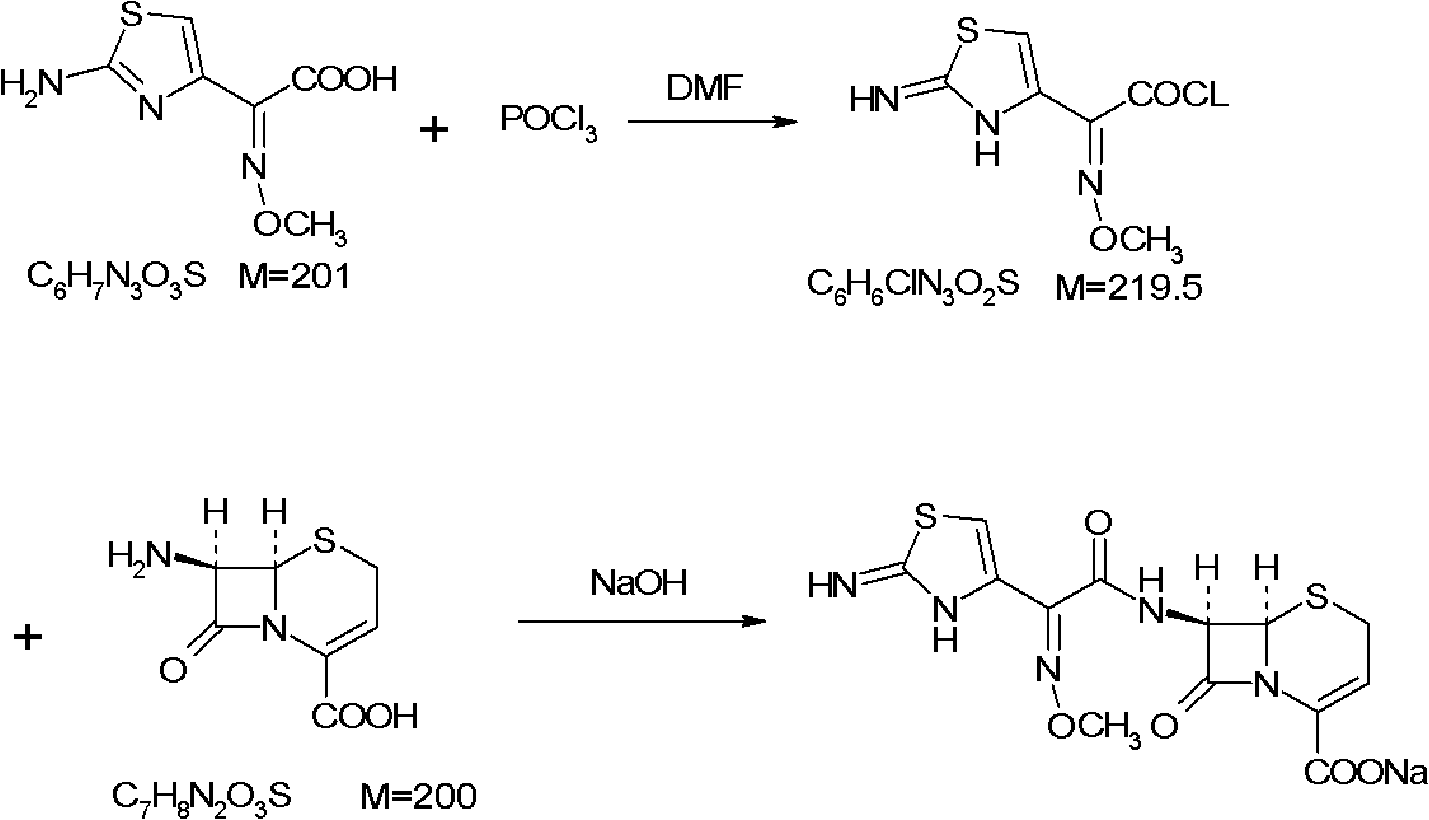
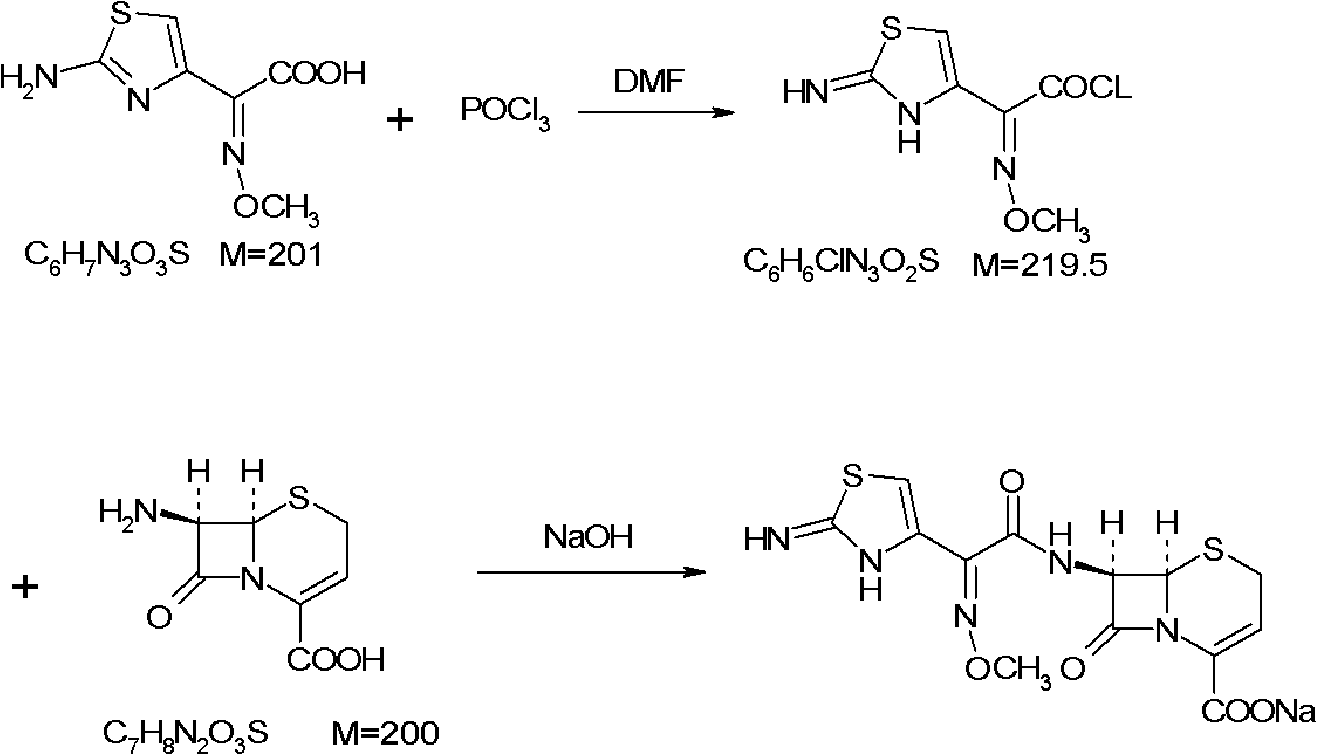
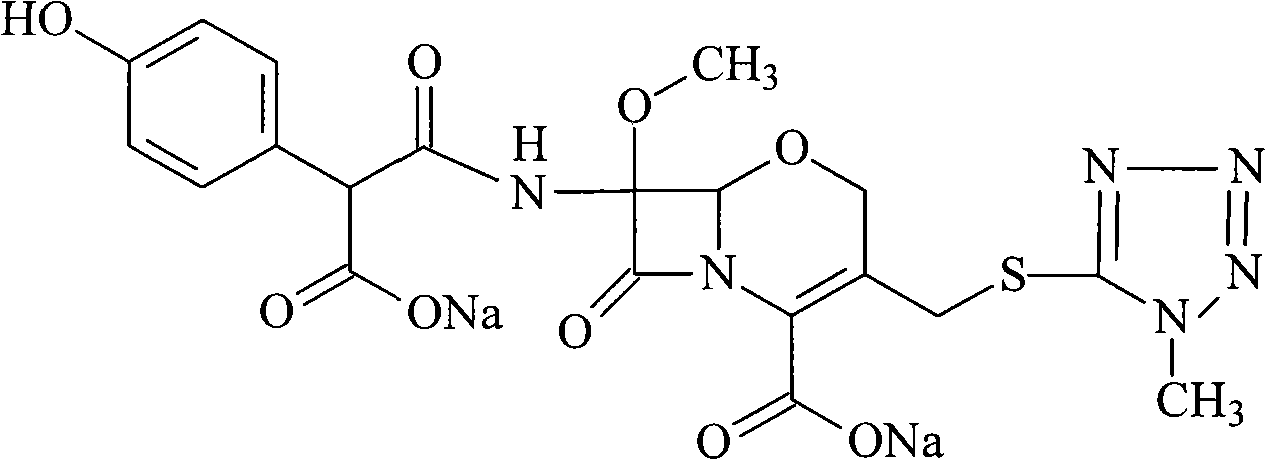
Ceftizoxime sodium drug injection powder and preparation method thereof, as well as synthetic method of bulk drug ceftizoxime sodium
ActiveCN101606910AUniform colorHigh purityAntibacterial agentsOrganic active ingredientsDrug injectionCLARITY
The invention relates to a ceftizoxime sodium drug injection powder and a preparation method thereof, as well as a synthetic method of bulk drug ceftizoxime sodium. The ceftizoxime sodium drug injection powder consists of 100% ceftizoxime sodium, wherein the ceftizoxime sodium is pretreated, and the pretreatment is aseptic refining and / or grinding. The ceftizoxime sodium drug has the advantages of high purity, almost no impurity, better and more stable quality, better clarity and the like; and the synthetic method of bulk drug ceftizoxime sodium has lower bulk cost, less synthesis technology difficulty, mild reaction condition, stable and reliable yield and quality, and high purity and yield of products.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Cefmetazole sodium medicament and preparation method thereof
ActiveCN101623285AReduce contentUniform colorAntibacterial agentsOrganic active ingredientsSolubilityMedicine
The invention relates to a cefmetazole sodium medicament and a preparation method thereof. The cefmetazole sodium medicament contains 100 percent of cefmetazole sodium which is preprocessed by sterile refining. Because the cefmetazole sodium is preprocessed by sterile refining, the powder fluidity of the cefmetazole sodium medicament is improved to be beneficial to separated packing, reduce the packing difference caused in the separated packing process and have simpler process and easy operation. The cefmetazole sodium sterile powder for injection prepared by the method has uniform color, high purity, almost no impurity, reduced stimulation, good solubility, faster redissolution, better clarity after redissolution and more stable quality and can be stored for a long time.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
Preparation method of human coagulation factor VIII
ActiveCN104231073ASuitable for a wide range of peopleHigh potencyFactor VIIPeptide/protein ingredientsAlcohol sugarsDiabetic patient
The invention discloses a preparation method of a human coagulation factor VIII. The human coagulation factor VIII prepared by the method does not contain human serum albumin or other animal-derived protein, does not contain sugar or sugar alcohol, does not have the risk for transmitting other viruses or pathogene, and is wide in applicable crowd scope, and can be used by diabetic patients; the human coagulation factor VIII prepared by the method is fast to redissolve and good in redissolving effect, and still keeps high titer and high specific activity which are respectively larger than 80 percent and 40 IU / mg; in addition, the preparation method is simple, the cost is low, the human coagulation factor VIII is safe and effective, and has a good industrial application prospect.
Owner:广东双林生物制药有限公司
Cefodizime sodium proliposome preparation and preparation method thereof
InactiveCN101584664AUnexpected effectNo toxicityAntibacterial agentsOrganic active ingredientsCefodizime SodiumCholesterol
The invention relates to a cefodizime sodium proliposome preparation and a preparation method thereof. The proliposome preparation comprises cefodizime sodium, egg yolk lecithin, cholesterol, antioxidant and supporting agent, wherein the cefodizime sodium proliposome preparation comprises the following components by weight portion: 1 to 20 portions of the cefodizime sodium, 5 to 50 portions of the egg yolk lecithin, 3 to 30 portions of the cholesterol, 0.5 to 20 portions of the antioxidant and 3 to 50 portions of the supporting agent.
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation method of fosaprepitant dimeglumine freeze-dried preparation for injection
InactiveCN102166199AProduct looseQuality improvementPowder deliveryOrganic active ingredientsPorosityFreeze-drying
A fosaprepitant dimeglumine freeze-dried preparation prepared by adopting the invention has the characteristics of porosity, stable quality, high redissolution speed, good clarity and the like. The invention provides a preparation method of a fosaprepitant dimeglumine freeze-dried preparation for injection, which comprises the following steps: (A) pre-freezing: firstly, cooling a fosaprepitant dimeglumine injection to (-12)-(-14) DEG C, and freezing through temperature oscillation; and then, cooling to (-32)-(-35) DEG C, and oscillating and freezing for 42-62 minutes; (B) sublimating: vacuumizing in a box body until the air pressure is 12-17 Pa, heating a product to (-35)-(-37) DEG C, and preserving the temperature for 8-10 hours; and intermittently injecting nitrogen gas, oscillating for 1-1.5 hours by taking air pressure (17-22 Pa) in the box body as an amplitude, heating the product to (-25)-(-29) DEG C, and preserving the temperature for 15-18 hours; and (C) drying: gradually heating the medicament to 38-42 DEG C, and preserving the temperature for 1-2 hours.
Owner:WUHAN LEADPHARM TECH CO LTD
Preparation method of terlipressin acetate lyophilized powder for injection
ActiveCN109223720AReduce moisture contentImprove stabilityPowder deliveryPeptide/protein ingredientsDrugs solutionMedicine
The invention relates to the pharmaceutical field, in particular to a preparation method of terlipressin acetate freeze-dried powder injection for injection. A method for prepare a terlipressin acetate lyophilized powder for injection comprises that following step of: pre-freezing the drug solution in stages, and then sequentially drying the drug solution in stages for the first time and drying the drug solution in stages for the second time. The invention can effectively improve the phenomenon of spraying bottle, and ensure that the prepared terlipressin acetate freeze-dried powder injectionhas good stability, complete sample appearance, rapid resolution and low moisture content.
Owner:南京康舟医药科技有限公司
Adenine arabinoside monophosphate freeze-dried powder injection and preparation method thereof
ActiveCN101642440ASimple operation processLow technical requirementsPowder deliveryOrganic active ingredientsEthylene diamineDrugs solution
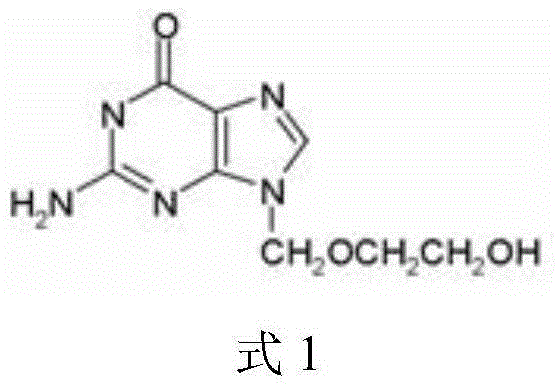
The invention relates to an adenine arabinoside monophosphate freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection is formed by freeze-drying an adenine arabinoside monophosphate drug solution. The adenine arabinoside monophosphate drug solution comprises 100g of adenine arabinoside monophosphate, 25g of mannitol, 3g of disodium hydrogen phosphate, 0.3gof monosodium orthophosphate, 1g of disodium ethylene diamine tetraacetate and 2,000ml of water for injection. The freeze-drying process in the preparation method comprises pre-freezing, sublimationand heating for drying. Compared with the common water injections in the prior art, the freeze-dried powder injection and the preparation method thereof have obvious advantages on storage and transportation.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
Terlipressin acetate injection and preparation method thereof
ActiveCN109010795AImprove stabilityReduce moisture contentPeptide/protein ingredientsDigestive systemDrugs solutionSample appearance
The invention relates to the field of pharmacy, in particular to a terlipressin acetate injection and a preparation method thereof. The preparation method of the terlipressin acetate injection comprises the following steps that a terlipressin acetate freeze-dried powder injection and a sodium chloride solution with the pH of 3.0 to 4.0 are mixed to obtain the injection, wherein after a medicine solution is subjected to staged pre-freezing, staged first drying and staged second drying are conducted in sequence. By means of the reparation method of the terlipressin acetate injection, the eruption phenomenon can be effectively improved, moreover, the fact that the prepared terlipressin acetate freeze-dried powder injection is good in stability, intact in sample appearance and capable of fastredissolving is guaranteed, meanwhile, the sodium chloride solution with the pH of 3.0 to 4.0 is adopted for redissolving, the injection stability is improved, and the safety and the effectiveness ofclinical medication are guaranteed.
Owner:南京康舟医药科技有限公司
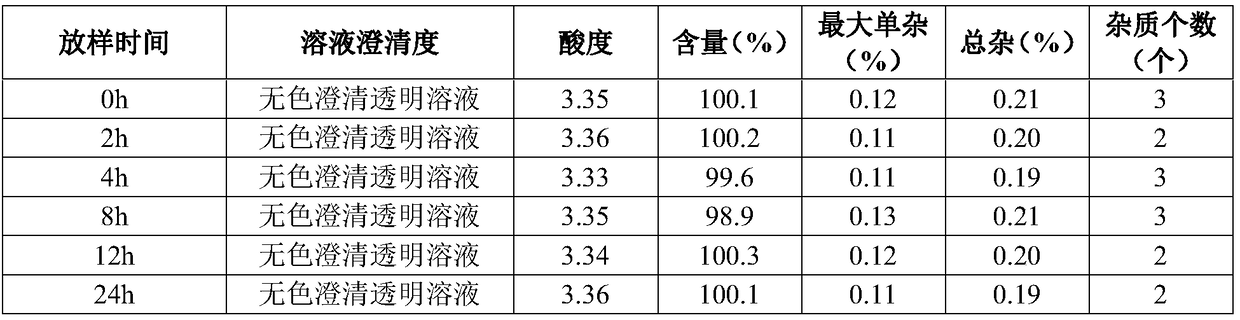
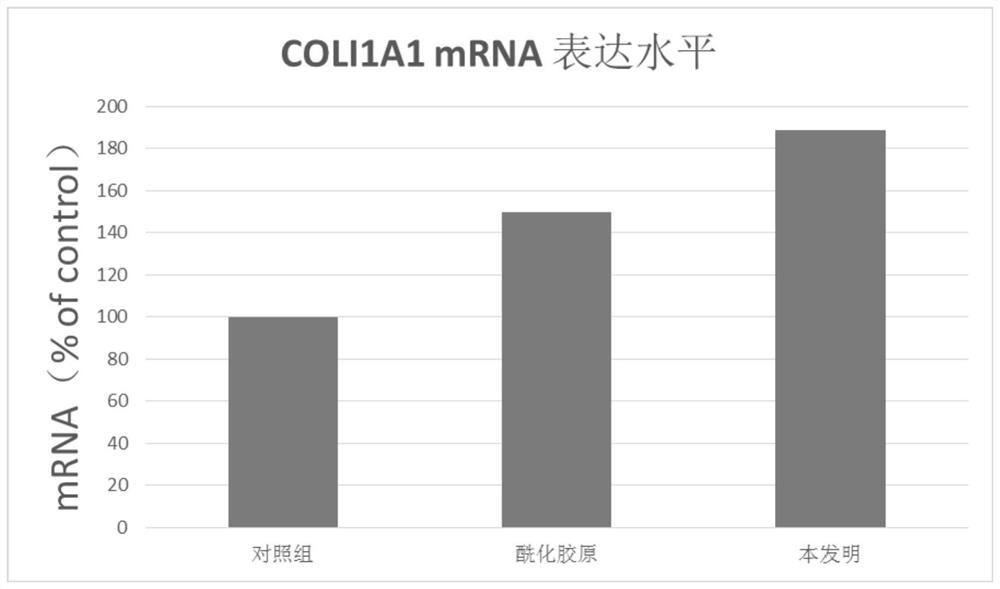
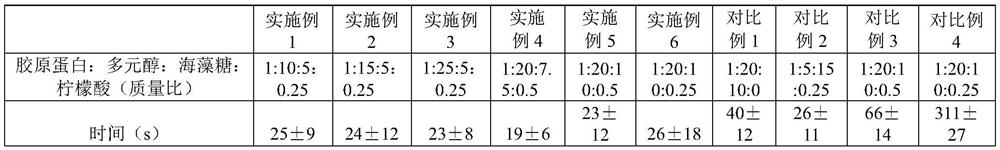
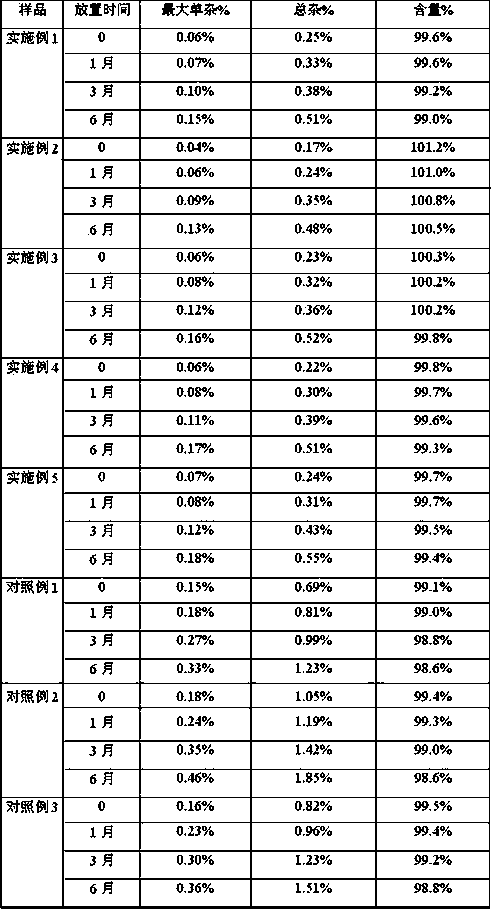
Active collagen lyophilized powder and preparation method thereof
ActiveCN111671667AImprove stabilityKeep natural activityCosmetic preparationsToilet preparationsPolyolFreeze-drying
The present invention discloses an active collagen lyophilized powder and a preparation method thereof. A rat tail collagen, polyols, mycose and citric acid are compounded, so that the stability of the rat tail collagen in a freeze-drying process may be jointly improved, a prepared lyophilized product may be stored without a preservative for a long time and is good in redissolution property, capable of keeping the natural bioactivity of collagen and remarkable in capability of promoting the synthesis of collagen in the skin.
Owner:GUANGDONG ZHONGERJIAN BIOTECH CO LTD
Bortezomib freeze-dried composition and preparation method thereof
InactiveCN103446068AShorten the dissolution timeReduce moisture contentPowder deliveryDipeptide ingredientsFreeze-dryingMannitol
The invention belongs to the field of medicine and provides a bortezomib freeze-dried composition and a preparation method thereof. The bortezomib freeze-dried composition is a freeze-dried composition which is obtained through the steps of spraying the mixed solution of bortezomib, mannitol and tertiary butanol into liquid nitrogen to form frozen microparticles, dissolving the microparticles in water for injection, and finally, performing freeze-drying. The bortezomib freeze-dried composition provided by the invention is better in quality and higher in stability.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Freeze dried monophosphate adenine arabinoside powder injection for injection and preparation method thereof
ActiveCN102389403ASimple operation processLow technical requirementsOrganic active ingredientsPowder deliveryDrugChemistry
The invention relates to freeze dried monophosphate adenine arabinoside powder injection for injection and a preparation method thereof. The freeze dried powder injection is prepared by freeze-drying a monophosphate adenine arabinoside liquid, wherein, the monophosphate adenine arabinoside liquid comprises 100 g of monophosphate adenine arabinoside, 25 g of mannitol, 3 g of disodium hydrogen phosphate, 0.3 g of sodium dihydrogen phosphate, 1 g of ethylenediaminetetraacetic acid, and 2000 mL water for injection. The preparation method comprises the following three steps: prefreezing, sublimating, and heating drying. Compared with common water injection in the prior art, the freeze dried powder injection and its preparation method disclosed herein have remarkable advantages in storage and transportation.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
Parecoxib sodium pharmaceutical composition for injection and preparation method thereof
The invention provides a parecoxib sodium pharmaceutical composition for injection and a preparation method thereof. The parecoxib sodium pharmaceutical composition for injection has the advantages that prescription of the parecoxib sodium pharmaceutical composition for injection is simple, dosage of disodium hydrogen phosphate is decreased greatly as compared with that of the prior art, and requirement for injection medicine safety is met well; since parecoxib sodium is in the shape of crystal, time for dissolution is shortened and time for dosing is then shortened, quality stability is guaranteed, and production efficiency is improved; by a rapid freezing process during preparation, free-dried products maintain good pore structure with quite uniform granularity which is favorable for complete sublimation of moisture; since a nitrogen charge operation is omitted, production procedure is simplified, cost is reduced, and socialized mass production is facilitated.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
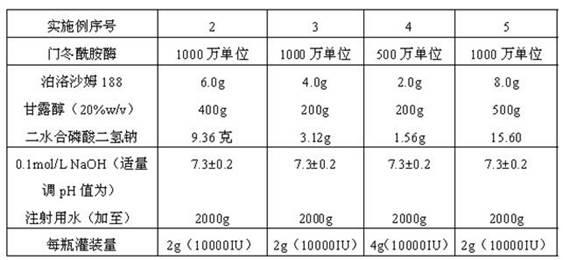
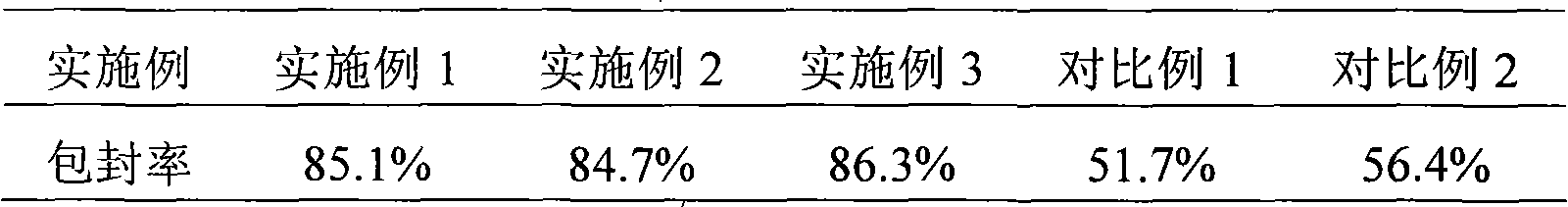
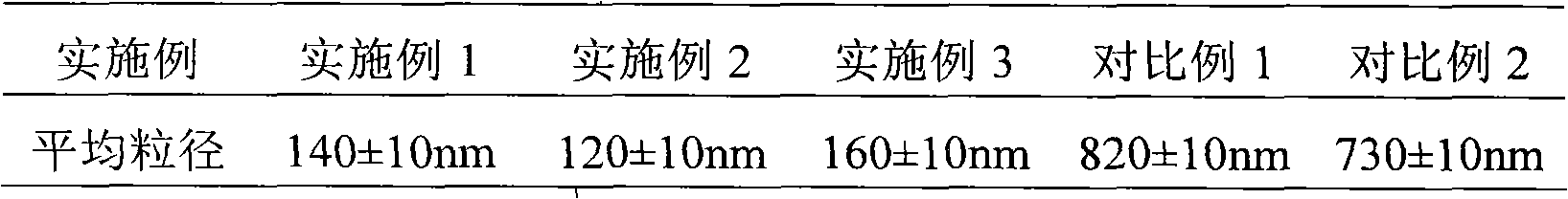
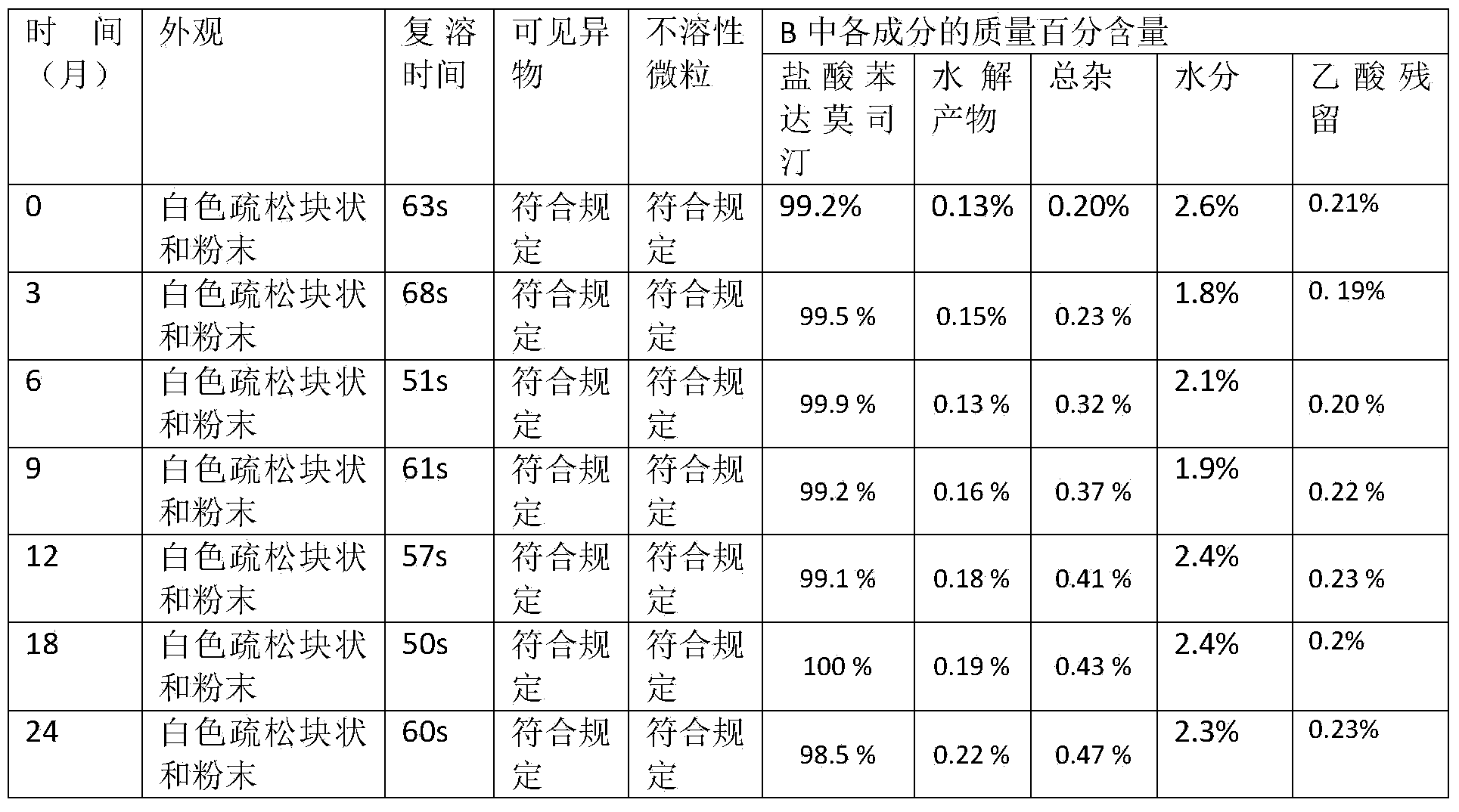
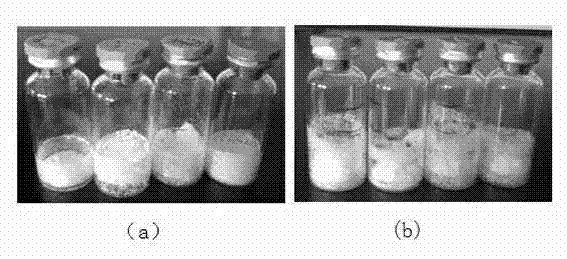
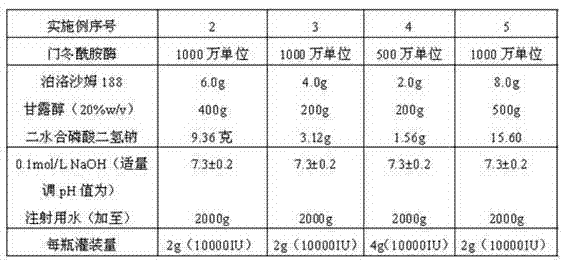
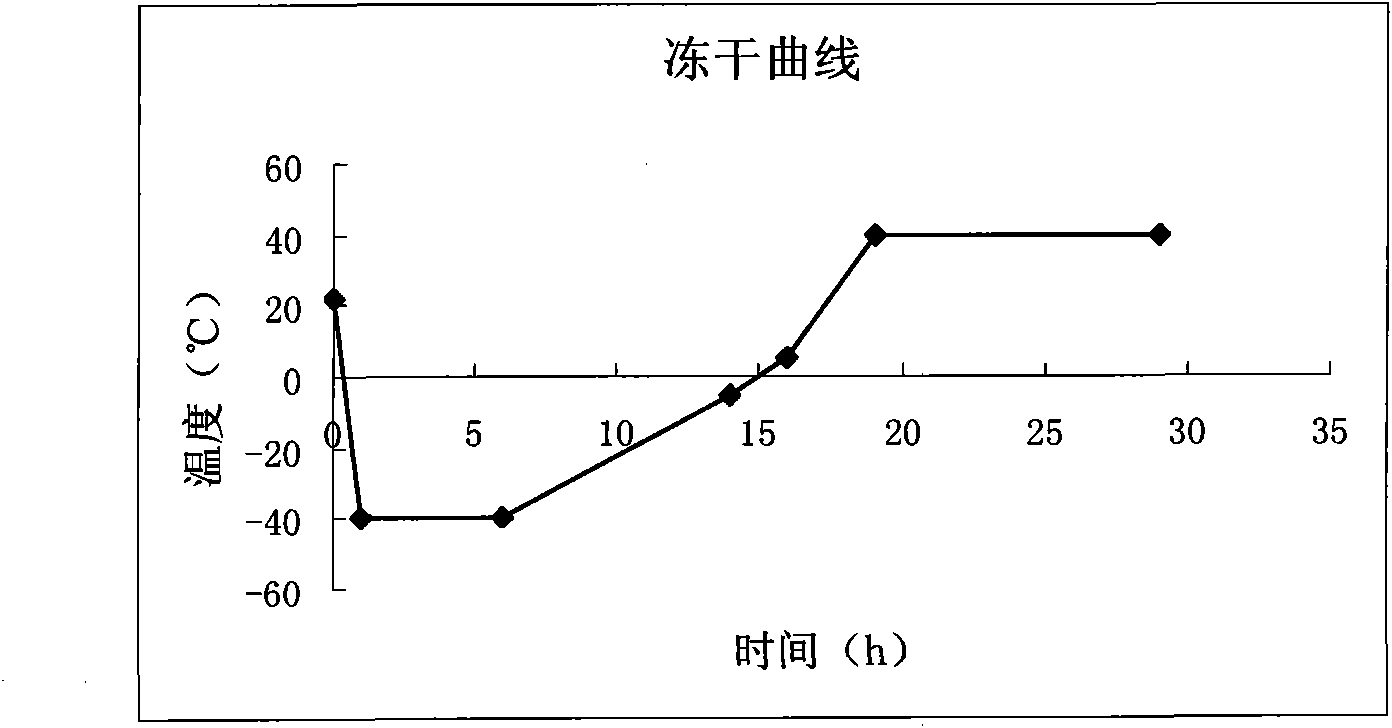
Asparaginase freeze-dried powder injection and preparation method thereof, as well as asparaginase solution
ActiveCN102138909AEasy to useRapid reconstitutionPowder deliveryPeptide/protein ingredientsFreeze-dryingSolvent
The invention discloses an asparaginase freeze-dried powder injection. The injection comprises asparaginase, a buffering agent, a surfactant, medical auxiliary materials and 5% of injection water which is used as a solvent in a preparing process and less than the addition finally, wherein the surfactant is a nonionic surfactant; and the buffering agent and the nonionic surfactant are added into the formula, so that the situation that tiny visible particles exceed standard is prevented when the asparaginase is dissolved and transferred into an injection. The invention also discloses a dedicated solution for dissolving the asparaginase, wherein the solution solvent is the injection water, and the solute comprises the buffering agent and the surfactant. On the premise of not changing the conventional formula of the freeze-dried powder injection, when the freeze-dried powder is dissolved in the solution, and is transferred into sodium chloride or a glucose injection, the situation that tiny visible particles exceed standard is prevented, so that the stimulation to human bodies is reduced in use, and the clinical administration safety is improved.
Owner:CHANGZHOU QIANHONG BIOPHARMA
Composition for injection containing fosaprepitant dimeglumine and preparation method thereof
ActiveCN104042572AThe process is simple and easy to controlReduce energy consumptionPowder deliveryOrganic active ingredientsDisodium EdetateChemistry
The invention provides a pharmaceutical composition containing fosaprepitant dimeglumine and a preparation method thereof. The pharmaceutical composition is composed of fosaprepitant dimeglumine, lactose, disodium edentate and tween-80, wherein a mass ratio among the fosaprepitant dimeglumine, the disodium edentate, the tween-80 and the lactose is 188-245.3:14.4-18.8:57.5-75:287.5-375. The composition is prepared in an aqueous containing alcohol in a freeze-drying manner. The preparation method is simple and controllable in processes and can reduce energy consumption. The pharmaceutical composition, prepared by the preparation method in the invention, is stable in quality and can ensure safety of clinical medication.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
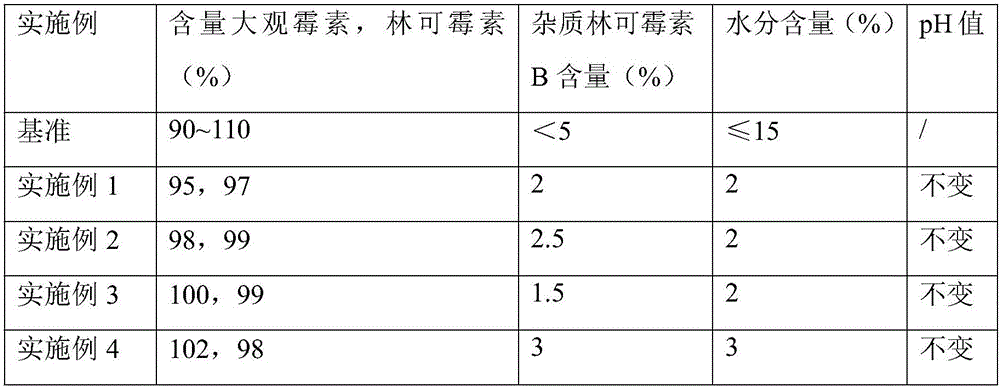
Spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection and preparation method of spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection
InactiveCN106420635AGood quality and stabilitySignificant effectPowder deliveryOrganic active ingredientsSpectinomycin HydrochlorideFreeze-drying
The invention relates to a spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection and a preparation method of the spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection, and solves the problems of high cost of industrial production and low drug stability in the prior art. The preparation method comprises the steps of firstly preparing a freeze-dried propping agent and antioxidant mixed solution, then preparing a to-be-freeze-dried sample of spectinomycin hydrochloride and lincomycin hydrochloride, and finally preparing the white loose block or powder spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection in a freeze drying box. According to the spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection and the preparation method of the spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection, the drug stability and the bioavailability are high, and the industrial production is realized.
Owner:LESHAN RECONDEX BIOPHARM CO LTD +1
Latamoxef sodium proliposome preparation
InactiveCN101642432AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsLatamoxefSide effect
The invention provides a latamoxef sodium proliposome preparation which comprises 1-20 portions of latamoxef sodium, 5-40 portions of di-stearic acid phosphatidyl choline, 2-15 portions of cholesteroland 3-50 portions of support agent. The invention also provides a preparation method and application of the proliposome preparation. The proliposome preparation has the advantages of high stability and entrapment efficiency, uniform particle diameter, less side effect, and the like.
Owner:HAINAN MEIDA PHARMA
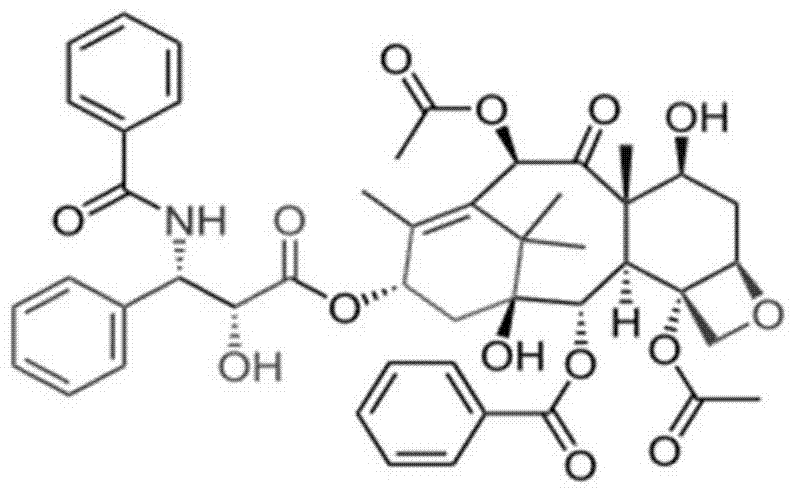
Excipient-containing lyophilized paclitaxel powder preparation and preparation method thereof
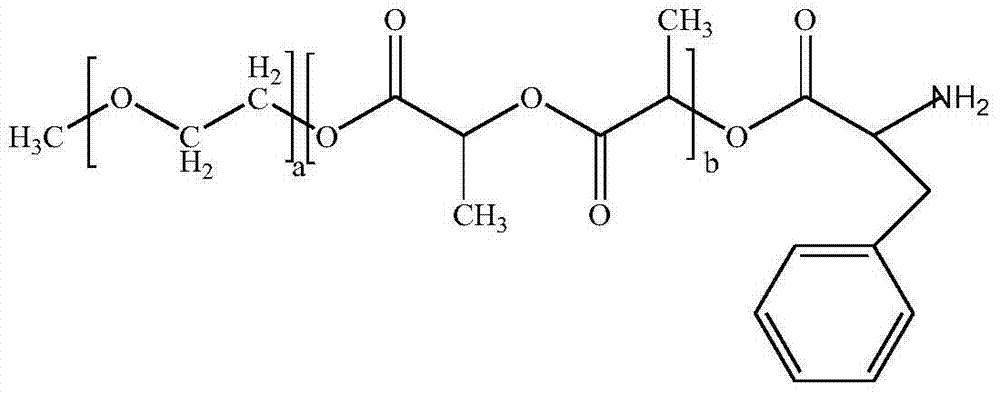
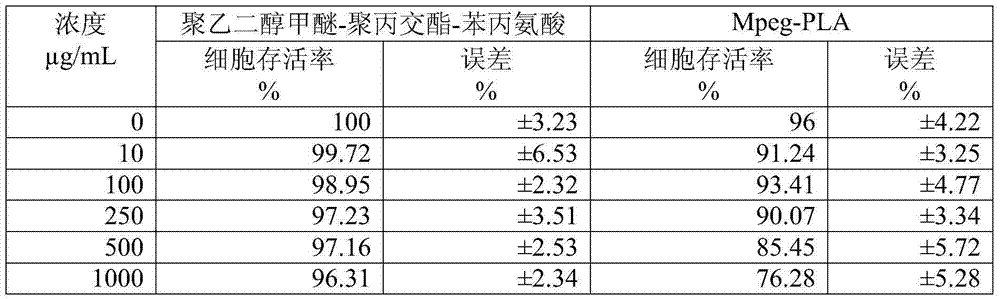
InactiveCN104510716ASimple preparation processSimple recipeOrganic active ingredientsPowder deliverySolubilityMixed micelle
The invention belongs to the technical field of medicinal preparations, and relates to an excipient-containing lyophilized paclitaxel powder preparation and a preparation method thereof. The preparation adopts paclitaxel as a medicinal effective component, adopts mPEG-PLA-phenylalanine as a matrix, and contains an excipient; and the excipient comprises one or more of lactose, mannitol, dextran, glycine and glucose. The preparation method comprises the following steps: 1, mixing mPEG-PLA-phenylalanine with paclitaxel; 2, fully dissolving the above obtained mixture in an organic solvent; 3, removing the organic solvent; 4, adding water, and hydrating to form a micelle solution; and 5, adding the excipient accounting for 5% or less of the total weight of materials added in step 1 to the medicine-loaded micelle solution, fully dissolving the excipient, lyophilizing the above obtained mixed micelle solution through a routine lyophilizing technology to prepare the excipient-containing lyophilized paclitaxel powder preparation. The excipient-containing lyophilized paclitaxel powder preparation has the advantages of simple preparation method, simple formula, high safety, good dissolution, rapid re-dissolving in water, and realization of small average particle size and easy performance of the ERP effect after the re-dissolving.
Owner:POLYMERCHEM
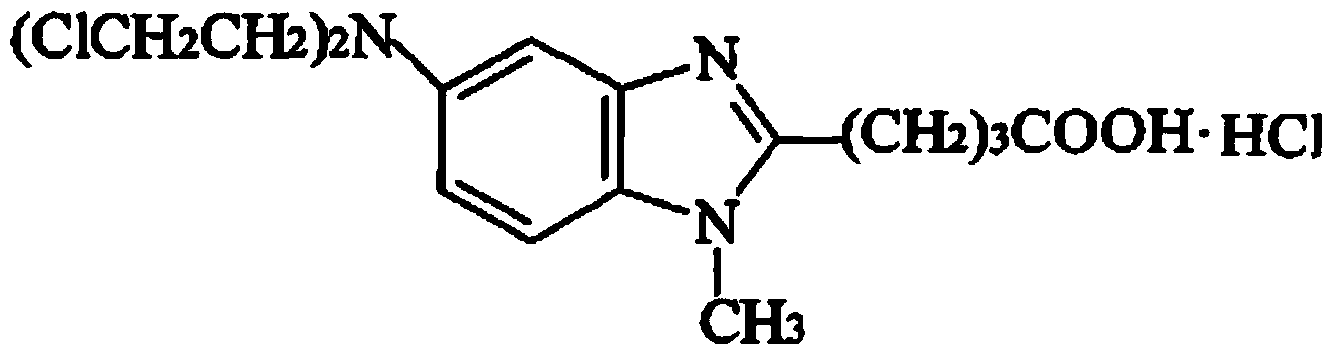
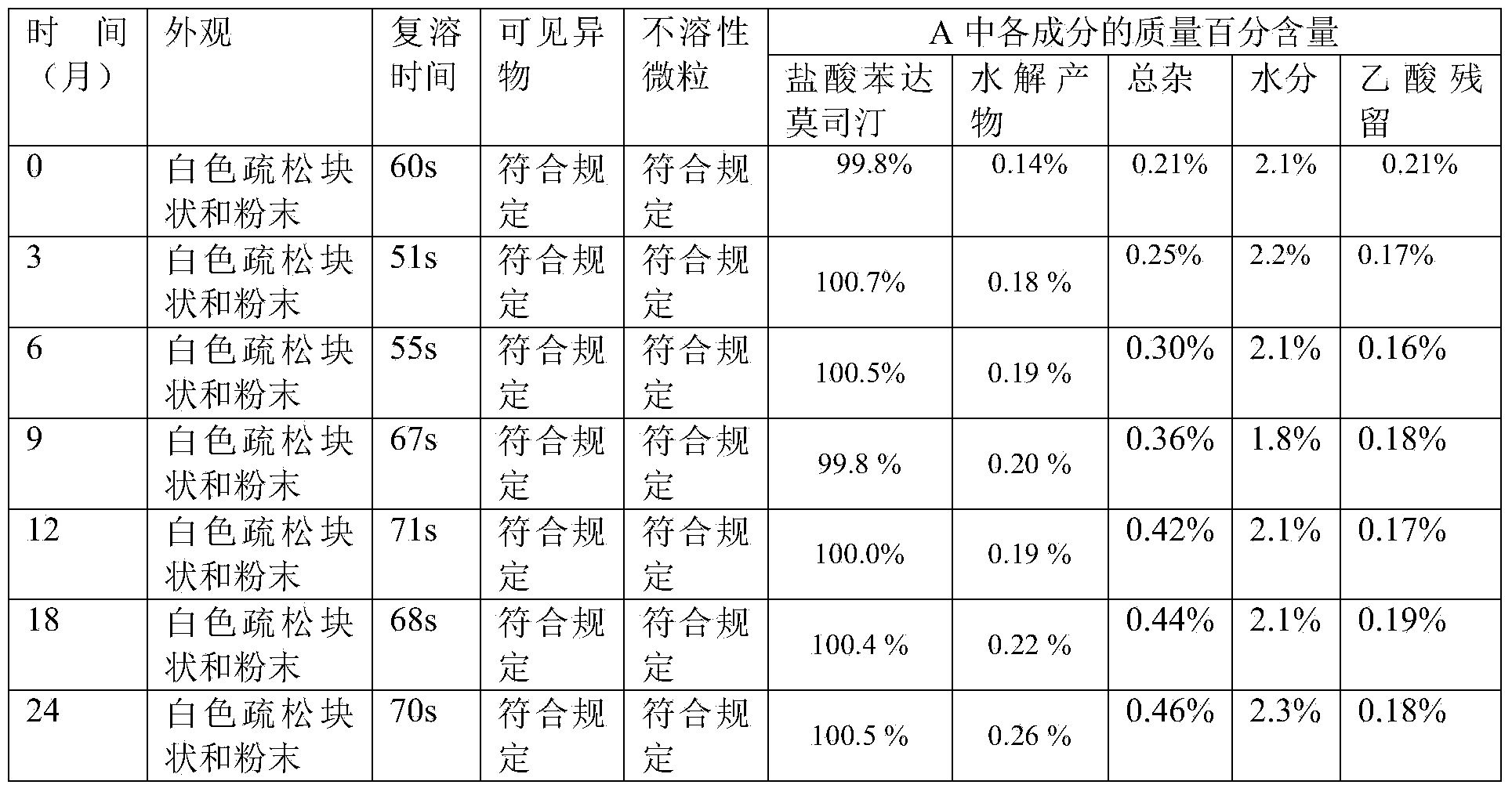
Preparation method of bendamustine hydrochloride freeze-dried injection
InactiveCN104173300ARapid reconstitutionOrganic active ingredientsPowder deliveryChemistryFreeze dry
The invention discloses a preparation method of a freeze-dried injection, and particularly relates to a preparation method of a bendamustine hydrochloride freeze-dried injection. The preparation method comprises the following steps: (1) dissolving filler into water for injection, so that a filler solution is obtained; (2) dissolving bendamustine hydrochloride into an acetic acid solution, so that a bendamustine hydrochloride-acetic acid solution is obtained; and (3) uniformly mixing the filler solution with the bendamustine hydrochloride-acetic acid solution, carrying out constant volume operation on the obtained mixture by using water for injection or an acetic acid solution at a temperature of 18-26 DEG C so as to obtain a bendamustine hydrochloride composition solution, and sequentially carrying out filtering, degerming and freeze drying on the bendamustine hydrochloride composition solution, so that the bendamustine hydrochloride freeze-dried injection is obtained. The preparation method disclosed by the invention is simple and easy to operate, and prepared products are good in stability and low in impurity content.
Owner:SICHUAN HUIYU PHARMA
Asparaginase freeze-dried powder injection and preparation method thereof, as well as asparaginase solution
ActiveCN102138909BEasy to useRapid reconstitutionPowder deliveryPeptide/protein ingredientsForeign matterFreeze-drying
The invention discloses an asparaginase freeze-dried powder injection. The injection comprises asparaginase, a buffering agent, a surfactant, medical auxiliary materials and 5% of injection water which is used as a solvent in a preparing process and less than the addition finally, wherein the surfactant is a nonionic surfactant; and the buffering agent and the nonionic surfactant are added into the formula, so that the situation that tiny visible particles exceed standard is prevented when the asparaginase is dissolved and transferred into an injection. The invention also discloses a dedicated solution for dissolving the asparaginase, wherein the solution solvent is the injection water, and the solute comprises the buffering agent and the surfactant. On the premise of not changing the conventional formula of the freeze-dried powder injection, when the freeze-dried powder is dissolved in the solution, and is transferred into sodium chloride or a glucose injection, the situation that tiny visible particles exceed standard is prevented, so that the stimulation to human bodies is reduced in use, and the clinical administration safety is improved.
Owner:CHANGZHOU QIANHONG BIOPHARMA
Preparation method for cerebroprotein hydrolysate freeze-dried injection for injection
ActiveCN101637457ASimple operation processThe technical content is not highPowder deliveryNervous disorderFreeze dryPhotochemistry
The invention relates to a preparation method for cerebroprotein hydrolysate freeze-dried injection for injection, which comprises the following steps: weighing 5000 volume of cerebroprotein hydrolysate solution; adding 4-10 mass / volume% of skeleton agent into the solution in the aseptic condition, and adjusting the range of Ph to be 6.9-7.5; collecting filter liquor after 0.45mum bacilli-eliminated filtration; filing and drying, wherein the drying time is sequentially 5 hours in the temperature of minus 40 DEG C, 4 hours in the temperature of minus 40 DEG C to minus 15 DEG C, 4 hours in the temperature of minus 15 DEG C to 5 DEG C, 2 hours in the temperature of minus 5 DEG C to 5 DEG C, 3 hours in the temperature of 5 DEG C to 40 DEG C, and 10 hours in the temperature of 40 DEG C; and thedrying time is totally 28 hours. The product prepared by the preparation method is stable and controllable; and the operational technique is simple.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
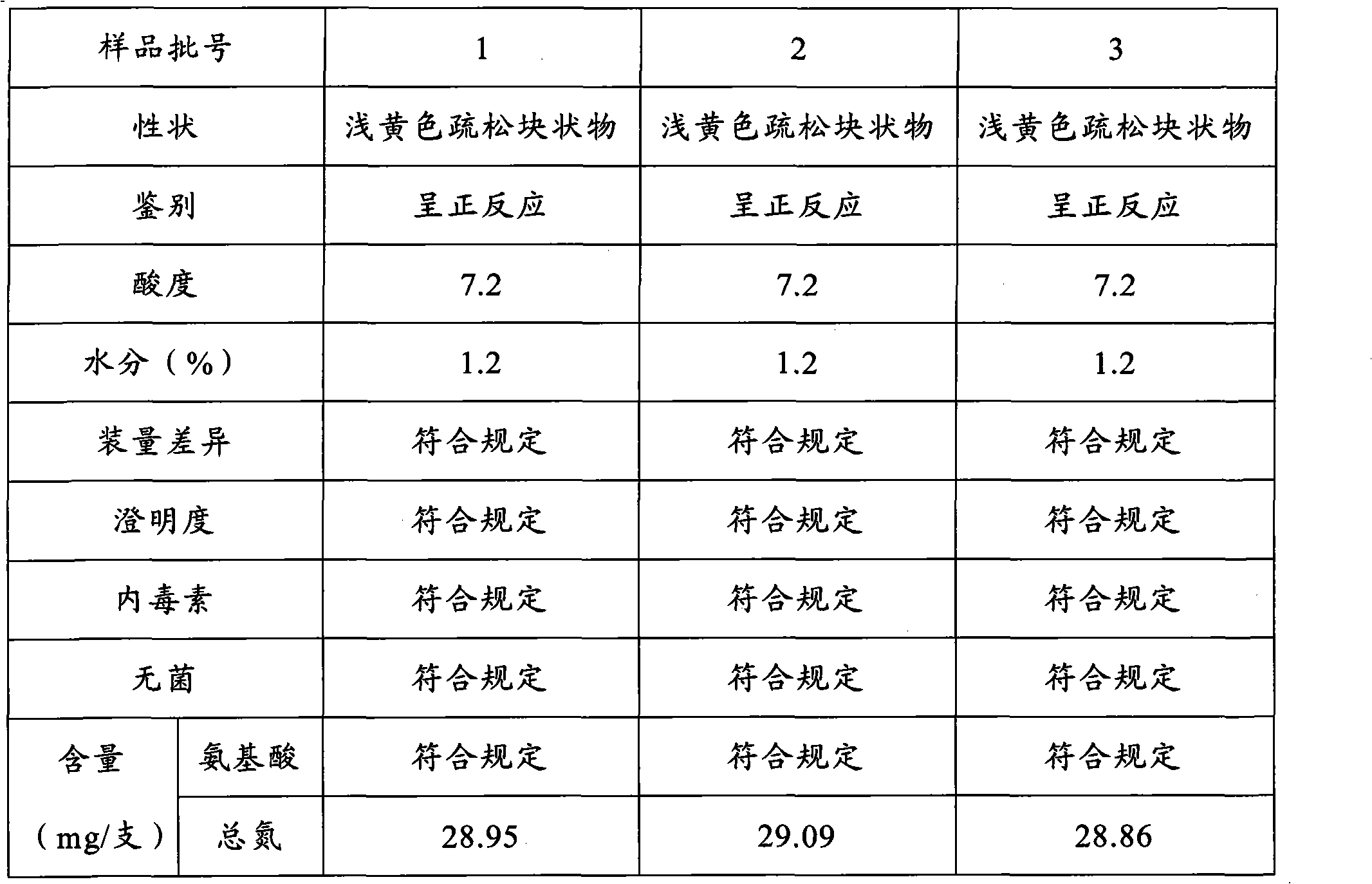
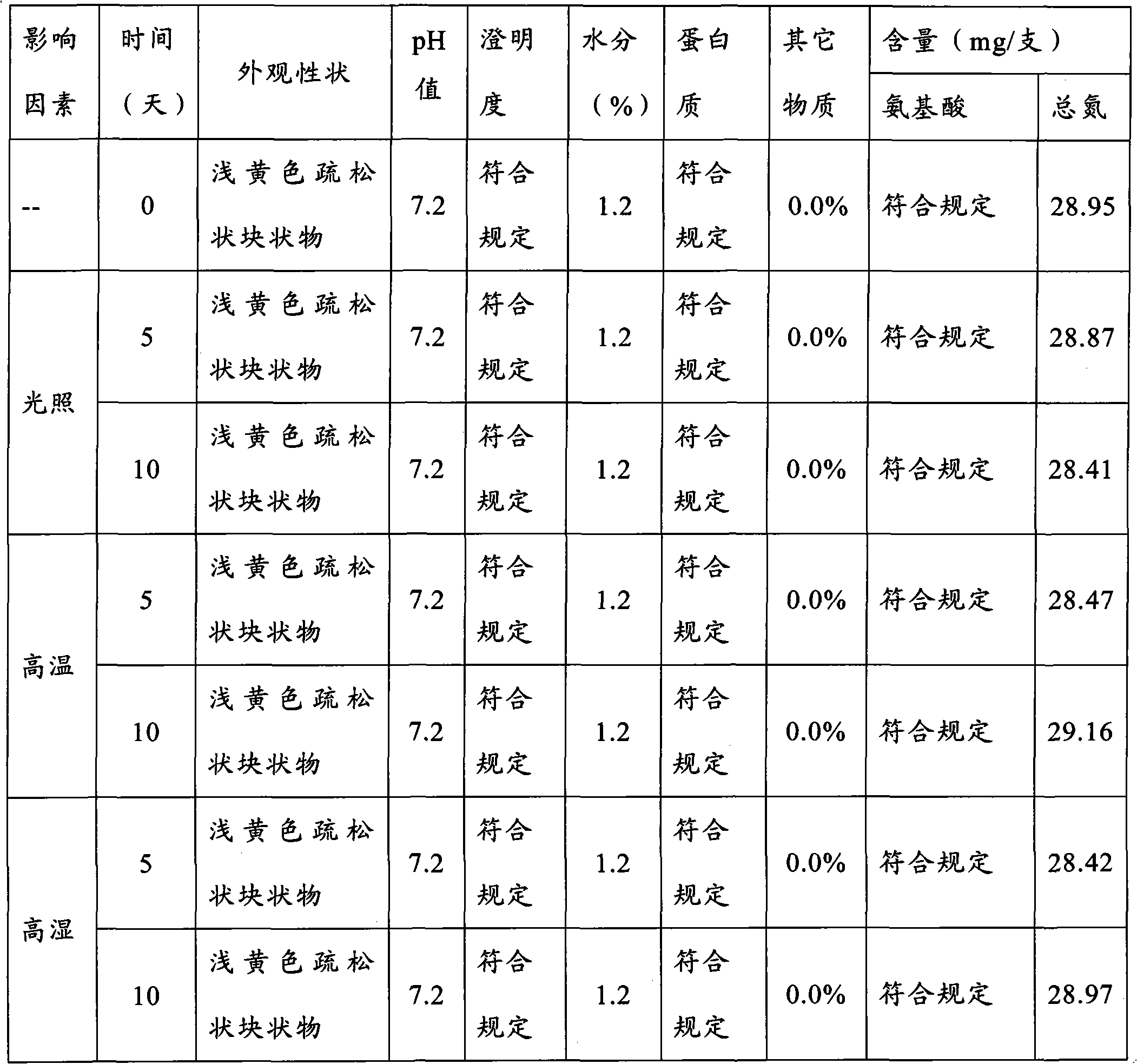
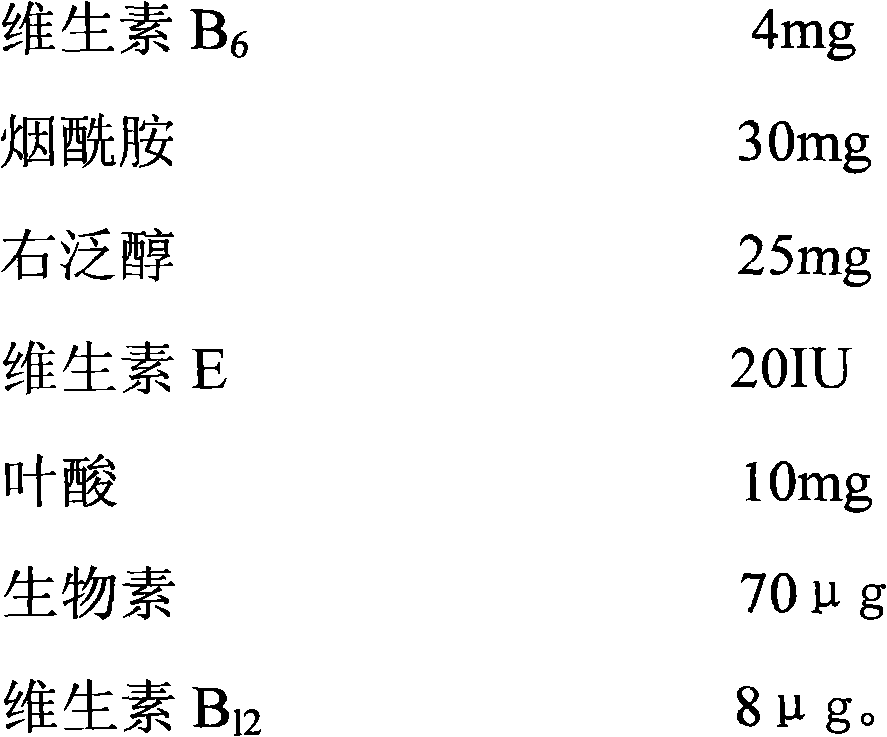
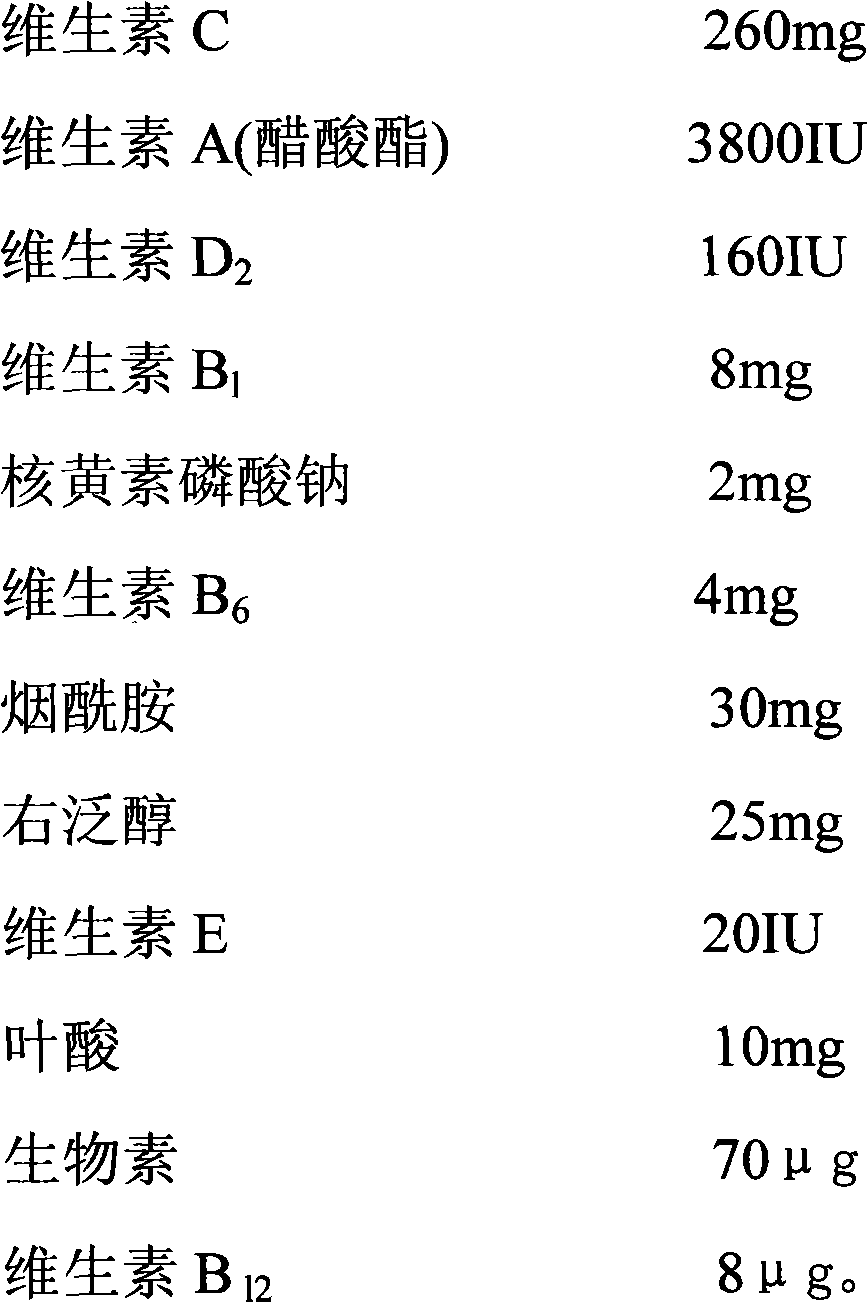
Preparation method of vitamin complex freeze-dried powder injection
InactiveCN102018720AFull appearanceRapid reconstitutionPowder deliveryHydroxy compound active ingredientsFreeze-dryingMultivitamin
The invention discloses a preparation method of a vitamin complex freeze-dried powder injection. The method comprises the following steps of: weighing 12 types of vitamins; adding a part of principle medicaments into polysorbate 80, stirring for dissolving, adding 40 percent water for injection and stirring for dissolving; adding the remaining principle medicaments into the solution, stirring for dissolving and replenishing water for injection to 80 percent of the total amount of the water for injection; measuring the pH of the solution and filtering by using a filter membrane; pre-freezing, namely, lowering the temperature of the injection to 15 DEG C below zero and oscillating and refrigerating at the temperature of between 6 DEG C below zero and 15 DEG C below zero; lowering the temperature to 33 DEG C below zero and 35 DEG C below zero and oscillating and refrigerating at the temperature of between 25 DEG C below zero and 35 DEG C below zero for 40 to 60 minutes; performing sublimation drying; drying once again; and gradually raising the temperature of a medicament to 40 DEG C and preserving heat for 1 to 2 hours. A product prepared by the method has full appearance, looseness, high dissolubility, quick re-dissolution, high clarity and high stability.
Owner:NORTH CHINA PHARMA COMPANY
Freeze-dry and dry heat treatment protecting agent for human blood coagulation factor VIII
ActiveCN104225601ASuitable for a wide range of peopleHigh potencyPharmaceutical non-active ingredientsActive agentAlcohol sugars
The invention discloses a freeze-drying and dry heat treatment protecting agent for human coagulation factor VIII. The freeze-drying and dry heat treatment protecting agent for human coagulation factor VIII does not contain human serum albumin or other animal-source proteins, sugar or sugar alcohol, is composed of soluble salt, amino acid and a surface active agent, is free from the risk of spreading other viruses or causative agents, and is applicable to a wide crowd range, including diabetics; after being freeze-dried, a product of the human coagulation factor VIII using the protecting agent is quick in redissolving and has a good redissolving effect, still keeps high potency and high specific activity, and has the potency higher than 80 percent and the specific activity higher than 40 IU / mg; in addition, the freeze-drying and dry heat treatment protecting agent for human coagulation factor VIII is low in cost, simple to prepare, has an obvious protecting effect, is safe and effective, and has an excellent industrial application prospect.
Owner:广东双林生物制药有限公司
Acyclovir lyophilized formulation for injection and preparation method acyclovir lyophilized formulation
InactiveCN104352458APlay a protective effectGood lookingPowder deliveryAntiviralsSolubilityMedical prescription
The invention relates to an acyclovir lyophilized formulation for injection and a preparation method of the acyclovir lyophilized formulation. The acyclovir lyophilized formulation for injection comprises acyclovir, sodium hydroxide, mannitol and a pH value stabilizing agent, wherein the weight ratio of the acyclovir to the mannitol is 1;(1-10). The acyclovir lyophilized formulation is advanced in prescription and good in product formability; the solution before being frozen is clear in appearance; the lyophilized product is good in solubility; and the re-dissolved solution is good in clarity, low in impurity content, good in stability and controllable in quality.
Owner:湖北潜龙药业有限公司
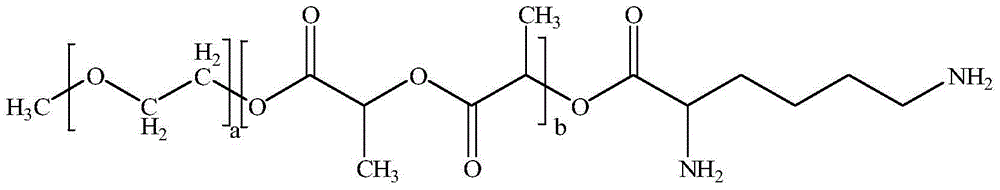
Excipient-free lyophilized paclitaxel powder preparation and preparation method thereof
InactiveCN104511024AEasy to prepareSimple recipeOrganic active ingredientsPowder deliveryHigh concentrationSolubility
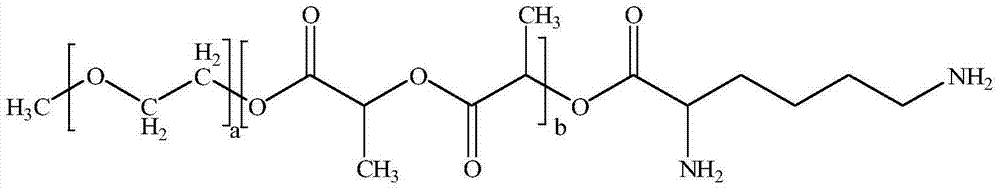
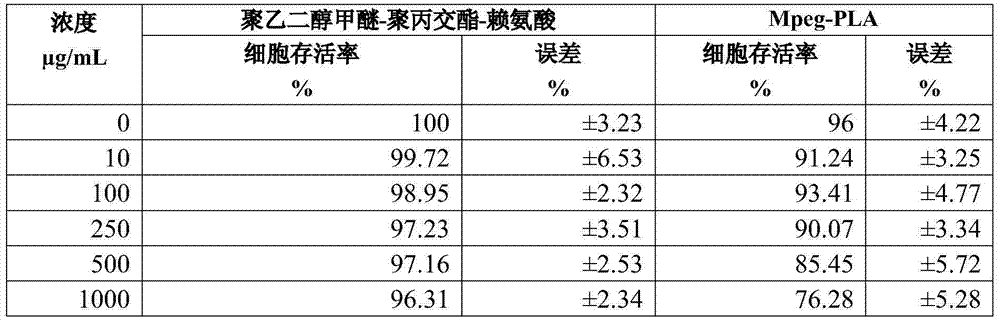
The invention belongs to the technical field of medicinal preparations, and relates to an excipient-free lyophilized paclitaxel powder preparation and a preparation method thereof. The lyophilized paclitaxel powder does not contain excipients, adopts paclitaxel as a medicinal effective component, and adopts mPEG- PLA-lysine as a matrix. The preparation method is simple; the excipient-free lyophilized paclitaxel powder preparation adopts mPEG-PLA-lysine with low cytotoxicityas as a carrier, and other auxiliary components are not needed, so the preparation has simple formula and high safety; the excipient-free lyophilized paclitaxel powder preparation has good solubility in water, can be rapidly re-dissolved in water, and has a highest concentration reaching 10mg / ml; and the re-dissolved lyophilized paclitaxel powder preparation has small average particle size, and easily performs an ERP effect.
Owner:POLYMERCHEM
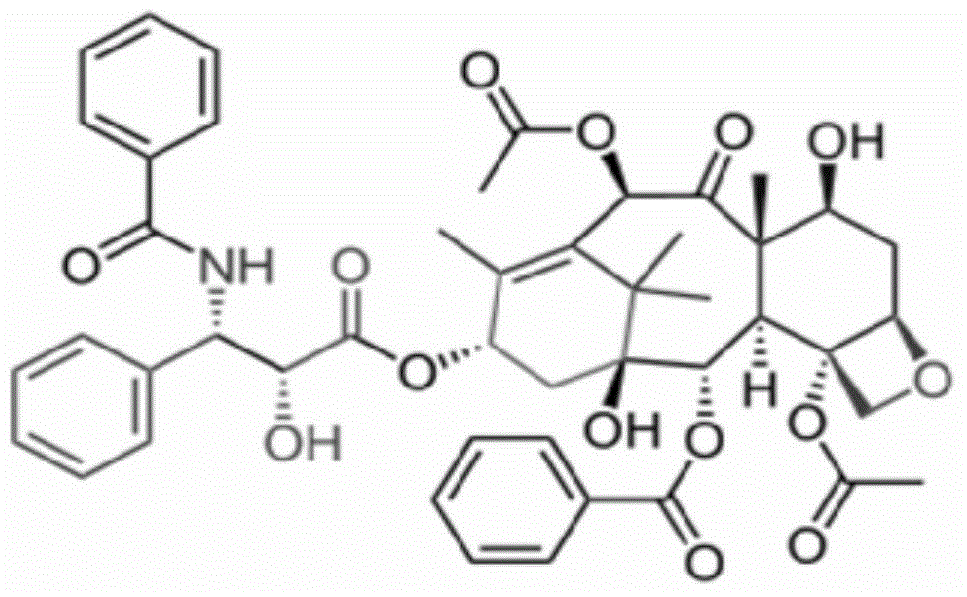
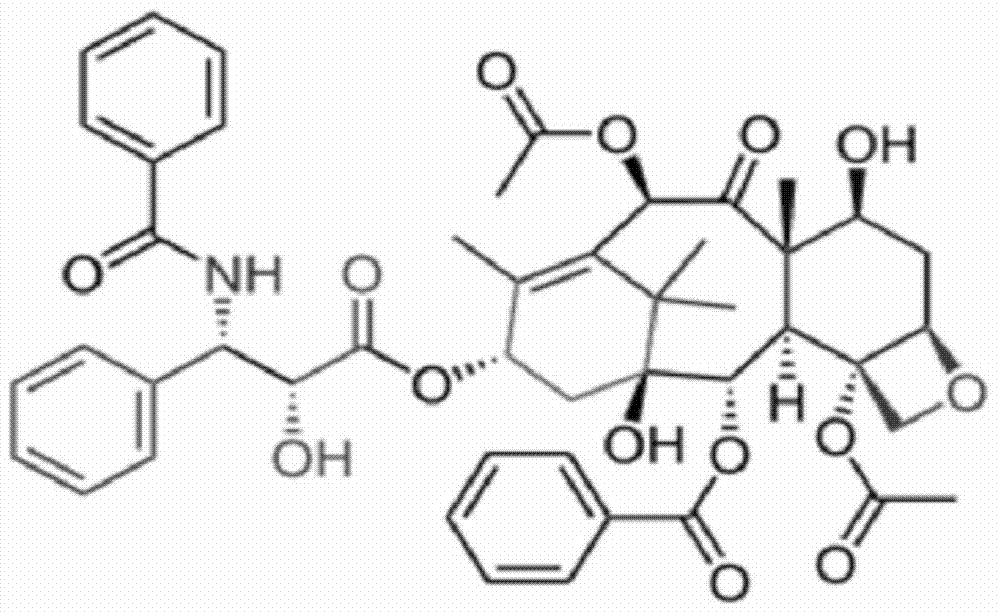
Taxol freeze-dried powder preparation containing excipient and preparation method of taxol freeze-dried powder preparation
InactiveCN104546740ASimple preparation processSimple recipePowder deliveryOrganic active ingredientsSolubilityFreeze-drying
The invention belongs to the technical field of pharmaceutical preparations, and in particular relates to a taxol freeze-dried powder preparation containing an excipient and a preparation method of the taxol freeze-dried powder preparation. The preparation takes taxol as a medicinal active ingredient and mPEG-PLA-lysine as a matrix and contains the excipient; and the excipient is selected from one or more of lactose, mannitol, dextran, glycine and glucose. The preparation method comprises the following steps: (1) mixing mPEG-PLA-lysine with taxol; (2) fully dissolving the mixture by using an organic solvent; (3) removing the organic solvent; (4) adding water for hydration to form a micellar liquid; and (5) adding the excipient of which the weight is not more than 5% of the total weight of the fed materials in the step (1) into the drug-loaded micellar liquid, dissolving the excipient fully, and then performing freeze-drying on the mixed micellar liquid according to a conventional freeze-drying process, thereby obtaining the taxol freeze-dried powder preparation. The preparation method provided by the invention is simple; the formula is simple, and the safety is high; and the freeze-dried powder preparation is good in solubility, can be re-dissolved quickly in water, has a relatively small average particle size after re-dissolution, and can give play to an ERP effect more easily.
Owner:POLYMERCHEM
Excipient-containing lyophilized paclitaxel powder preparation and preparation method thereof
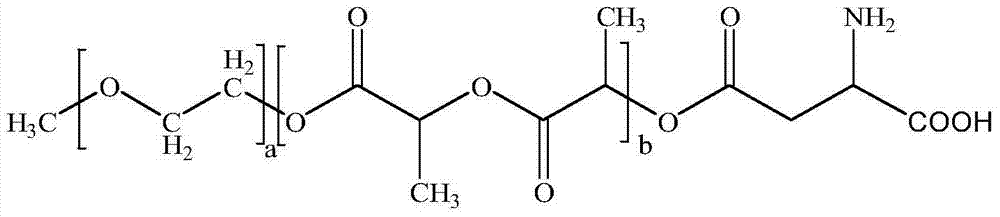
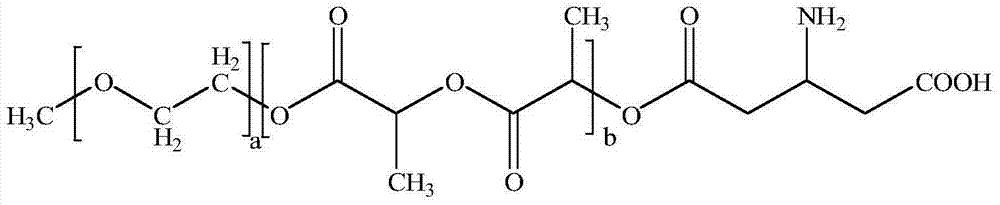
InactiveCN104510715ASimple preparation processSimple recipePowder deliveryOrganic active ingredientsSolubilityMixed micelle
The invention belongs to the technical field of medicinal preparations, and relates to an excipient-containing lyophilized paclitaxel powder preparation and a preparation method thereof. The preparation adopts paclitaxel as a medicinal effective component, adopts mPEG-PLA-aspartic acid as a matrix, and contains an excipient; and the excipient comprises one or more of lactose, mannitol, dextran, glycine and glucose. The preparation method comprises the following steps: 1, mixing mPEG-PLA-aspartic acid with paclitaxel; 2, fully dissolving the above obtained mixture in an organic solvent; 3, removing the organic solvent; 4, adding water, and hydrating to form a micelle solution; and 5, adding the excipient accounting for 5% or less of the total weight of materials added in step 1 to the medicine-loaded micelle solution, fully dissolving the excipient, lyophilizing the above obtained mixed micelle solution through a routine lyophilizing technology to prepare the excipient-containing lyophilized paclitaxel powder preparation. The excipient-containing lyophilized paclitaxel powder preparation has the advantages of simple preparation method, simple formula, high safety, good dissolution, rapid re-dissolving in water, and realization of small average particle size and easy performance of the ERP effect after the re-dissolving.
Owner:POLYMERCHEM
Excipient-free lyophilized paclitaxel powder preparation and preparation method thereof
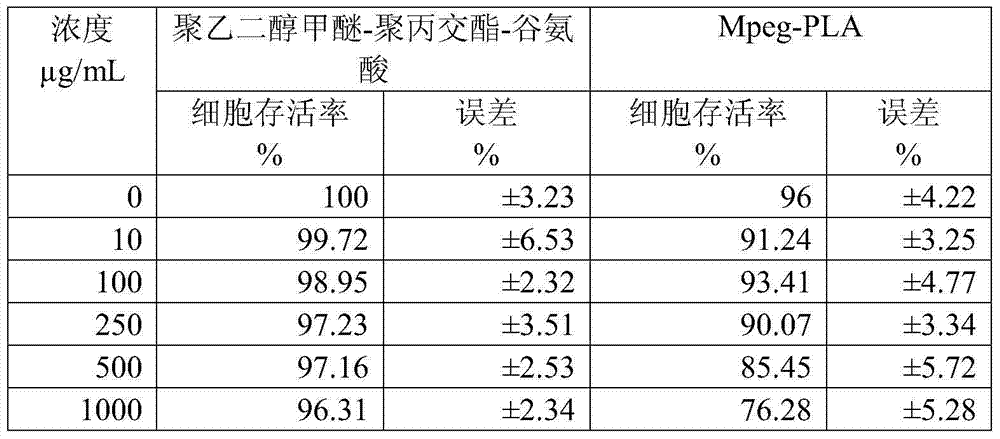
InactiveCN104510712AEasy to prepareSimple recipeOrganic active ingredientsPowder deliverySolubilityMedicine
The invention belongs to the technical field of medicinal preparations, and relates to an excipient-free lyophilized paclitaxel powder preparation and a preparation method thereof. The lyophilized paclitaxel powder does not contain excipients, adopts paclitaxel as a medicinal effective component, and adopts mPEG- PLA-glutamic acid as a matrix. The preparation method is simple; the excipient-free lyophilized paclitaxel powder preparation adopts mPEG-PLA-glutamic acid with low cytotoxicityas as a carrier, and other auxiliary components are not needed, so the preparation has simple formula and high safety; the excipient-free lyophilized paclitaxel powder preparation has good solubility in water, and can be rapidly re-dissolved in water; and the re-dissolved lyophilized paclitaxel powder preparation has small average particle size, and easily performs an ERP effect.
Owner:POLYMERCHEM
Multivitamin parenteral nutritional agent nanosphere freeze-dried injection and preparation method thereof
ActiveCN109010292ARapid reconstitutionConvenient for clinical applicationPowder deliveryHydroxy compound active ingredientsWater solubleFat-Soluble Vitamin
The invention discloses a multivitamin parenteral nutritional agent nanosphere freeze-dried injection, which is prepared from thirteen vitamins, medium chain triglyceride, soybean lecithin, 15-polyethylene glycol hydroxystearate, glycerin, mannitol and water, wherein the mass ratio of the soybean lecithin to the 15-polyethylene glycol hydroxystearate is equal to 1 to (1.4-4), and the mass ratio ofthe weight of the medium chain triglyceride to the total weight of the soybean lecithin and the 15-polyethylene glycol hydroxystearate is equal to 1 to (1-4). The fat-soluble vitamins are made into alipid nanosphere solution, and the lipid nanosphere solution can be co-dissolved with a water-soluble vitamin solution, so that the problem of co-dissolution of the fat-soluble vitamins and water-soluble vitamins is ingeniously solved. The multivitamin parenteral nutritional agent nanosphere freeze-dried finished product provided by the invention is clear transparent liquid after being redissolved with water, and can be diluted with an aqueous solution infinitely. The product is high in safety and quality, good in stability, easy in operation of the production process, and low in production cost.
Owner:CHINESE MEDICINES GUANGZHOU
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com