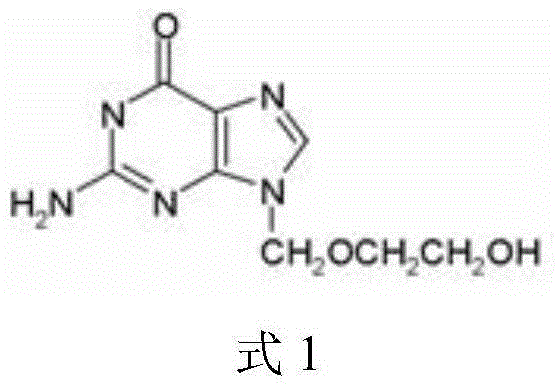
Acyclovir lyophilized formulation for injection and preparation method acyclovir lyophilized formulation
A technology for freeze-dried preparations and injections, which is applied in the direction of freeze-dried transportation, medical preparations of non-active ingredients, antiviral agents, etc. It can solve problems such as difficulty in ensuring long-term stability, achieve good clarity, low impurity content, Quality Controlled Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of acyclovir freeze-dried preparation for injection
[0036]
[0037] Mix acyclovir and mannitol, add water for injection, add sodium hydroxide, stir until completely dissolved, add 1.5% ethanol by volume, and adjust the pH value to 11.0 with sodium acetate, add activated carbon for needles 0.01g / 100ml, stir , filtered and decarbonized, the medicinal solution is finely filtered with a 0.22-micron sterile microporous membrane to obtain acyclovir sterile medicinal solution, which is then subpackaged, semi-stoppered, freeze-dried, plugged, and capped to obtain freeze-dried Finished preparations.
[0038] Wherein, freeze-drying step is:
[0039] (1) Pre-freezing: Vacuumize the cabinet to 15 Pa, lower the temperature of the acyclovir sterile medicinal solution to -15 degrees Celsius under vacuum conditions, and freeze for 15 minutes; remove the vacuum, then cool down to -40 degrees Celsius, and freeze for 40 minutes. minute;
[0040](2) Sublimatio...
Embodiment 2
[0042] Example 2 Preparation of acyclovir freeze-dried preparation for injection
[0043]
[0044] Mix acyclovir and mannitol, add water for injection, add sodium hydroxide, stir until completely dissolved, add 2.0% ethanol by volume, and adjust the pH value to 11.2 with sodium phosphate, add 0.02g / 100ml of activated carbon for needles, and stir , filtered and decarbonized, the medicinal solution is finely filtered with a 0.22-micron sterile microporous membrane to obtain acyclovir sterile medicinal solution, which is then subpackaged, semi-stoppered, freeze-dried, plugged, and capped to obtain freeze-dried Finished preparations.
[0045] Wherein, freeze-drying step is:
[0046] (1) Pre-freezing: Vacuumize the cabinet to 10 Pa, lower the temperature of the sterile acyclovir liquid to -20 degrees Celsius under vacuum conditions, and freeze for 15 minutes; remove the vacuum, then cool down to -40 degrees Celsius, and freeze for 60 minute;
[0047] (2) Sublimation drying: v...
Embodiment 3
[0049] Example 3 Preparation of Acyclovir Lyophilized Formulation for Injection
[0050]
[0051] Mix acyclovir and mannitol, add water for injection, add sodium hydroxide, stir until completely dissolved, add 1.5% ethanol by volume, and adjust the pH value to 11.5 with sodium dihydrogen phosphate, add 0.02g / 100ml of activated carbon for needles , stirred, filtered and decarbonized, and the medicinal liquid was finely filtered with a 0.22 micron sterilizing microporous membrane to obtain acyclovir sterile medicinal liquid, which was then subpackaged, semi-stoppered, freeze-dried, plugged, and capped to obtain The finished freeze-dried preparation.
[0052] Wherein, freeze-drying step is:
[0053] (1) Pre-freezing: Vacuumize the cabinet to 15 Pa, lower the temperature of the sterile acyclovir liquid to -18 degrees Celsius under vacuum conditions, and freeze for 12 minutes; remove the vacuum, then cool down to -40 degrees Celsius, and freeze for 40 minutes. minute;
[0054...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com