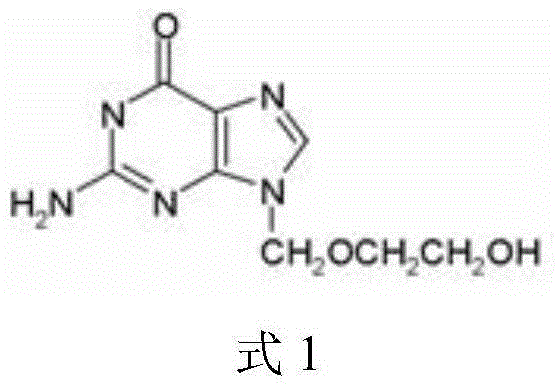
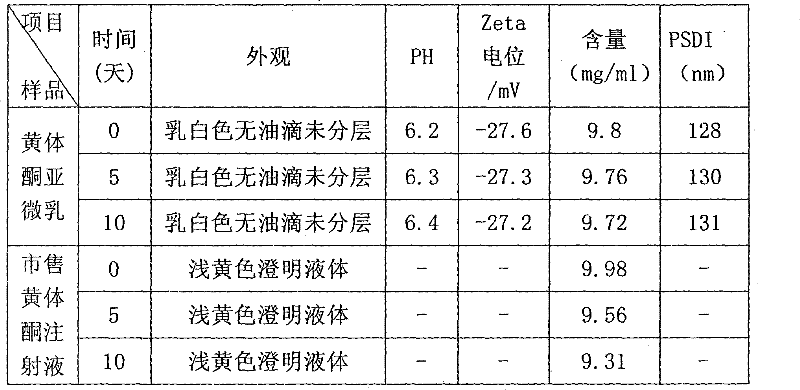
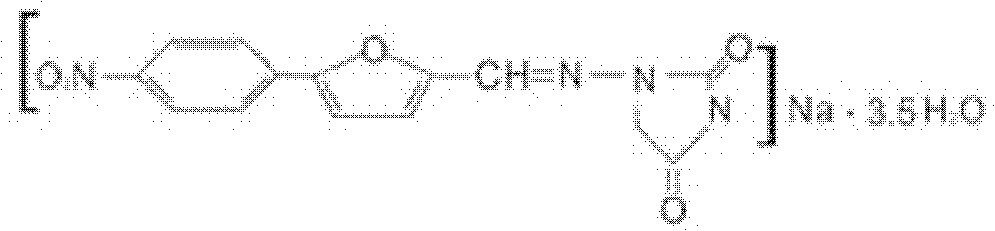
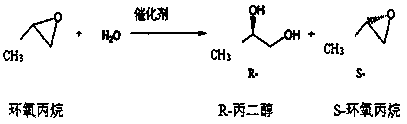Patents
Literature
43results about How to "Appearance clarification" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lornoxicam freeze-dried injection and preparation method thereof
ActiveCN101327193AHigh clarityGood formabilityPowder deliveryOrganic active ingredientsMedicineFreeze-drying
The invention relates to lomoxicam freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection comprises omoxicam, mannite, tromethamine, EDTA and pH regulator. The dosage of the mannite is 3.5 to 8.5 g of mannite per gram of lomoxicam, and the dosage of the EDTA is 0.015 to 0.025g of EDTA per gram of lomoxicam. The invention adopts EDTA to replace EDTA-disodium salt and selects the dosages of the mannite and EDTA so that the clarity of the reconstituted obtained freeze-dried powder is greatly improved, and the formability is good; in addition, the invention adopts two pH regulators, in the process, one pH regulator is used to regulate the solution to a pH range, then the other pH regulator is used to regulate the solution to another pH range, and the freeze drying technology is controlled strictly. The prepared freeze-dried powder injection has great improvement of stability and favorable resolubity.
Owner:HAINAN JINRUI PHARMA CO LTD
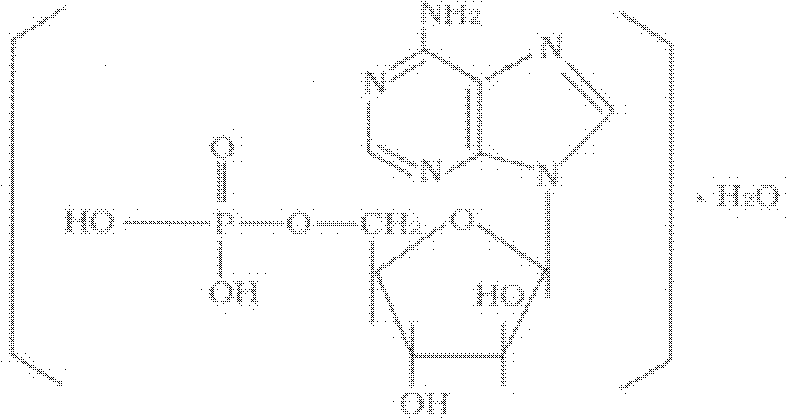
Vidarabine monophosphate freeze-dried powder injection and preparation method thereof
ActiveCN102379853AGood formabilityHigh clarityPowder deliveryOrganic active ingredientsFreeze-dryingPhosphoric acid
The invention relates to a vidarabine monophosphate freeze-dried powder injection and a preparation method thereof, and the vidarabine monophosphate freeze-dried powder injection is prepared by freeze-drying vidarabine monophosphate, sodium hydroxide solution and water for injection, wherein the sodium hydroxide solution is used as a pH regulator, and the sodium hydroxide solution is used until the pH value of liquid medicine is 7.0-7.5. The vidarabine monophosphate freeze-dried powder injection provided by the invention is few in types of auxiliary materials, low in using amount, good in forming property of a freeze-dried product, good in re-dissolving property, clear in appearance of the solution before freezing, good in clarity after being freeze-dried, low in content of impurities, good in stability and controllable in quality, and can reduce the potential safety hazard of the vidarabine monophosphate freeze-dried powder injection, and improve the efficacy of the vidarabine monophosphate freeze-dried powder injection.
Owner:HAINAN JINRUI PHARMA CO LTD
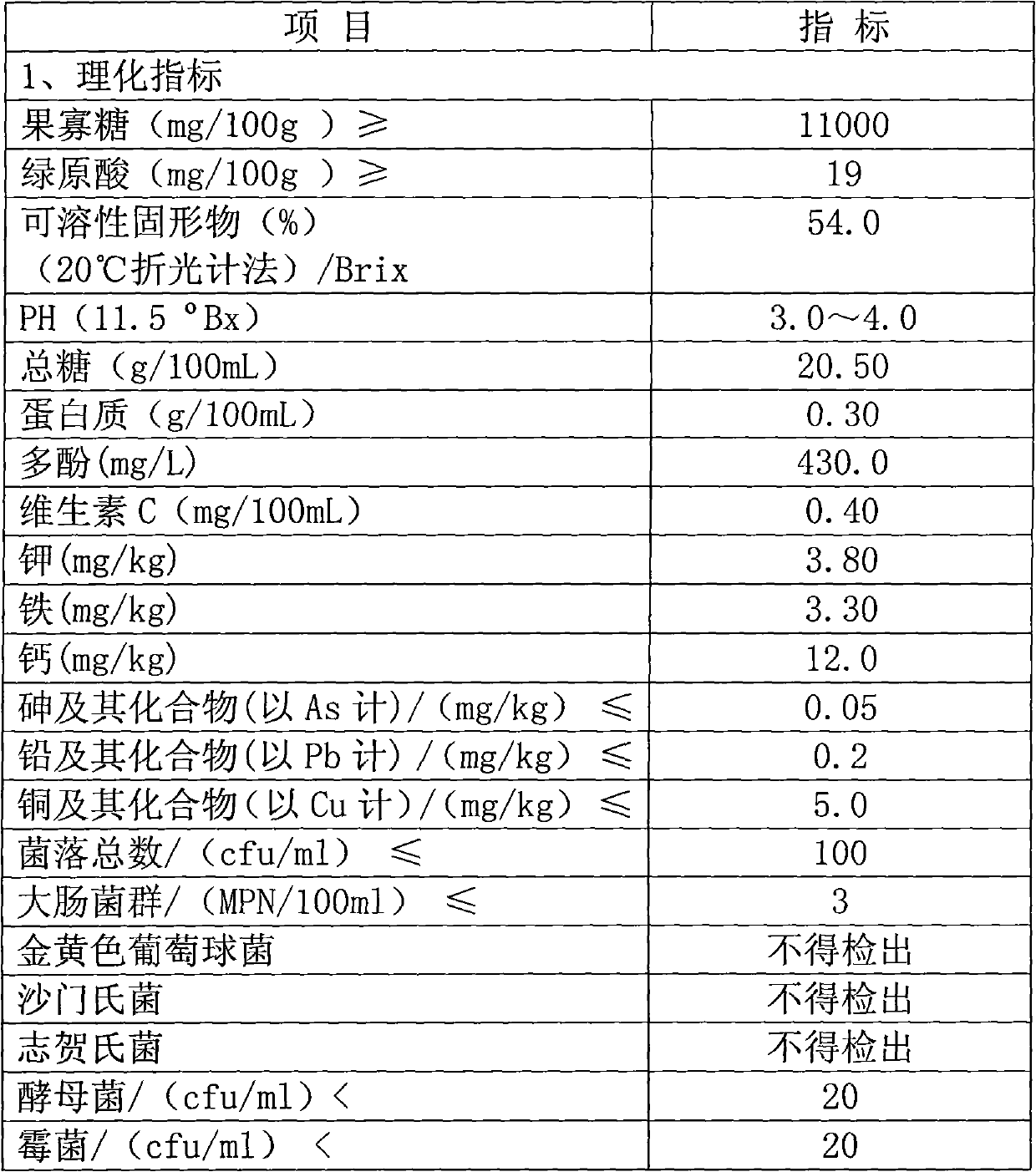
Double-component yacon fruit juice and preparation method thereof
The invention provides yacon fruit juice and a preparation method thereof. The yacon fruit juice contains fructooligosaccharide and chlorogenic acid, wherein the content of the fructooligosaccharide is not less than 11,000mg / 100g, and the content of the chlorogenic acid is not less than 19mg / 100g. The preparation method for producing the yacon fruit juice comprises that yacon tubers are prepared into a yacon fruit juice product by cleaning, peeling, crushing, color protection, juicing, enzyme treatment, adsorption, filtration, centrifugation, blending, high-temperature sterilization and filling. The yacon fruit juice keeps the nutrient components of the fructooligosaccharide and the chlorogenic acid, ensures that the contents of the fructooligosaccharide and the chlorogenic acid do not decline along with prolonging of storage time, and also keeps the unique flavor of yacon fruits. The guarantee period of the product is 1 year, and the yacon fruit juice has no sediment or suspension and does not change color in the guarantee period.
Owner:贵阳高新创嘉创业服务有限公司
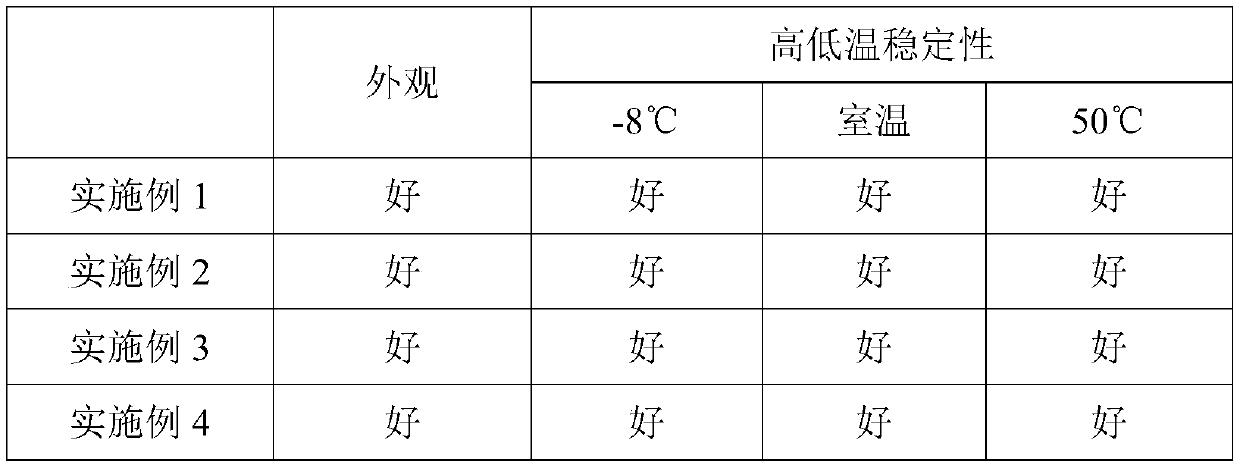
No-clean disinfection mousse composition
InactiveCN111249169AGood dispersionImprove wettabilityCosmetic preparationsToilet preparationsGlycineAlcohol ethyl
The invention relates to the technical fields of disinfection and sterilization, and more specifically relates to a no-clean disinfection mousse composition. The composition uses a novel material N-alkylpropylaminoglycine, the content and ratio of ethanol and the N-alkylpropylaminoglycine are adjusted, the foaming speed and foam tightness are adjusted, so that the dispersibility, wettability and drying speed of the no-clean mousse based on N-alkylpropylaminoglycine on hands are greatly improved, and the use sense and acceptance of the product are improved; the formula is simple, the productionprocess is convenient, a large amount of ethanol does not need to be added, and the safety factor in the production process is high; and in addition, the appearance is clear without turbidity or precipitation, has excellent high and low temperature stability, and does not show delamination, discoloration, precipitation and other phenomena when stored for 3 months at the environments of -8 DEG C,room temperature and 50 DEG C.
Owner:上海绿瑞生物科技有限公司
Changshanhuyou tangelo and Quzhouhertao peach compound juice drink and preparation method
InactiveCN103584225ASweet and sour tasteWeaken or eliminate fire-clearing effectMulti-step food processesFood ingredient functionsBiotechnologyFRUIT PUNCH
The invention discloses a Changshanhuyou tangelo and Quzhouhertao peach compound juice drink, and belongs to the technical field of drinks and preparations of the drinks. The drink is prepared by components in parts by weight as follows: 10-12 parts of Changshanhuyou tangelo juice, 20-24 parts of Quzhouhertao peach juice, 5-7 parts of liquorice extracting solutions, 5-7 parts of red autumn sunflower extracting solutions and the balance of water, wherein the total number of parts of the total volume of the drink is 100. The provided compound juice drink has tempting color and sour, sweet and mouthwatering taste, no edible essence and pigment are added, and the original characteristics of raw materials are kept to the greatest extent; and the compound juice drink has the efficacy of decreasing internal heat, replenishing strength and keeping health and is suitable for people of all ages and both sexes.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Preparation method for monosialotetrahexosyl ganglioside sodium injection
ActiveCN102988284AAppearance clarificationLess impuritiesOrganic active ingredientsNervous disorderActivated carbonWater baths
The invention relates to a preparation method for a monosialotetrahexosyl ganglioside sodium injection. The method comprises the following steps of 1) weighing disodium hydrogen phosphate, sodium dihydrogen phosphate and sodium chloride, adding half amount of injection water, heating to a temperature of 40 DEG C to make the above material dissolved, and thus an auxiliary material solution is obtained; 2) weighing a raw material of monosialotetrahexosyl ganglioside sodium, adding into the above auxiliary material solution, and stirring until the is completely dissolved; 3) adding 20 g of activated carbon, stirring for 30 minutes, and filtering out the activated carbon; 4) supplementing the injection water until the volume of the injection water is 19 L, adjusting a pH value to 7.4-7.6 by using sodium hydroxide or hydrochloric acid, and supplementing the injection water until the volume of the injection water is 20 L; and 5) sterilizing for 20 minutes at the temperature of 121 DEG C by using rotary water bath sterilization after loading.
Owner:哈药集团股份有限公司 +1
Dantrolene sodium freeze-dried powder injection for injection and preparation method thereof
ActiveCN102302461AImprove toleranceDosing time is shortPowder deliveryOrganic active ingredientsForeign matterCLARITY
The invention belongs to the technical field of medicines, and particularly relates to a dantrolene sodium freeze-dried powder injection for injection and a preparation method thereof. The freeze-dried powder injection is prepared by freeze drying dantrolene sodium, lactobiose, a pH regulator and water for injection, wherein a mass ratio of the dantrolene sodium to the lactobiose is (1:0.5) to (1:2.5). The preparation method comprises the following steps of: adding the dantrolene sodium to the lactobiose in a prescription amount into the water for the injection, stirring, regulating the pH value to 9.0 to 10.5 by using the pH regulator, adding active carbon for injection, removing a heat source, decolorizing, filtering for decarburizing, performing fine filtering by using a filter membrane, packaging in separate bags, freezing and drying. The dantrolene sodium freeze-dried powder injection is studied on the basis of a freeze-dried process, namely the dantrolene sodium freeze-dried powder injection is cooled, heated by a small margin and is cooled again, so that the moisture of the freeze-dried product is reduced, and the freeze-dried product is high in redissolution and visible foreign matters and does not have insoluble granules. The dantrolene sodium freeze-dried powder injection for the injection is high in resolubility, clarity and stability, and low impurity content.
Owner:HAINAN JINRUI PHARMA
Soda water beverage and preparation method of same
InactiveCN106360200AExtended shelf lifeImprove nutritional qualityFood ingredient as taste affecting agentNatural extract food ingredientsNutrientSodium bicarbonate
The invention discloses a soda water beverage. Each 1000 L of the soda water beverage comprises, by weight, 0.10-0.20 kg of sodium bicarbonate, 0.2-0.4 kg of citric acid, 0.10-0.20 kg of vitamin C, 0.01-0.02 kg of sucralose and 8-20 kg of calamansi juice. The soda water has long shelf life and abundant nutrients, and satisfies demands of common people, and has great advantages on improving body immunity and enhancing body physique.
Owner:贵州北极熊实业有限公司
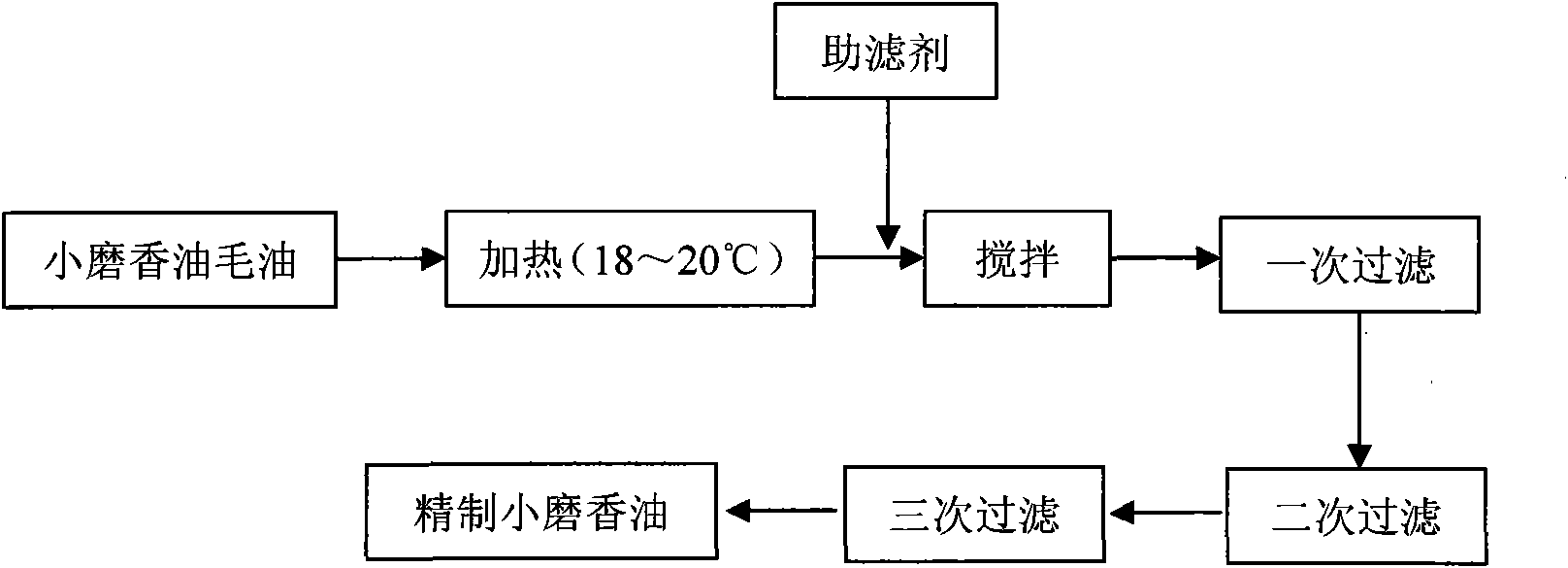
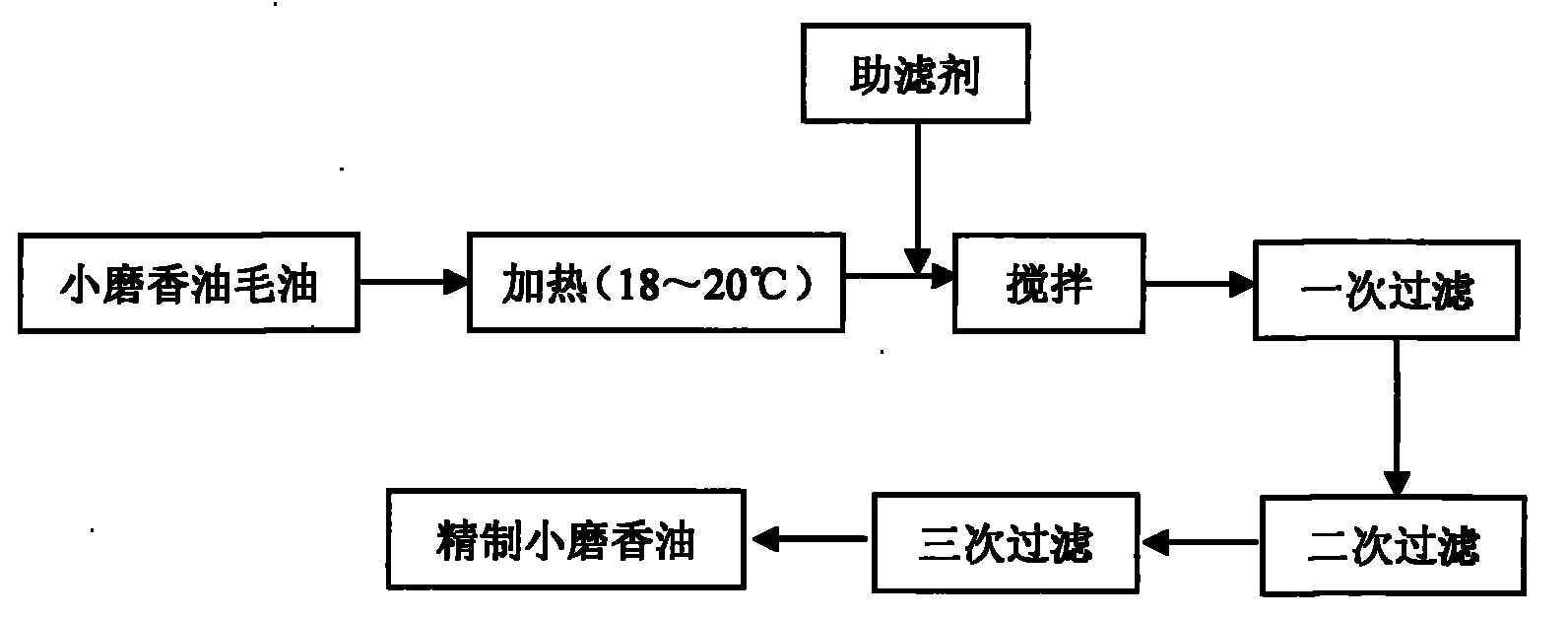
Ground sesame seed oil fine filtering method
InactiveCN101843280AReduce moistureLow impurity contentEdible oils/fats production/working-upPlant fibreEngineering
The invention discloses a ground sesame seed oil fine filtering method, belonging to the food field. The method comprises heating crude ground sesame seed oil produced through a water substitution process to approximately 18-20 DEG C first, adding 1-2 percent of natural filter aid and conducting primary filtration through a vibrating residue-discharge filter after even agitation; after primary filtration, conducting secondary natural sedimentation filtration by using a natural plant fiber substance as a filter screen; and conducting tertiary filtration through a cloth bag filter to obtain refined ground sesame seed oil product finally. The ground sesame seed oil produced through the method of the invention has the advantages of low water content and impurity content, high clarity and transparency, stable product quality, long quality guarantee period, short production time, simple technology, low cost and easy industrialization; and the method can be used for the production of high-quality grinding sesame seed oil.
Owner:冀中能源邢台矿业集团有限责任公司油脂分公司
Acyclovir lyophilized formulation for injection and preparation method acyclovir lyophilized formulation
InactiveCN104352458APlay a protective effectGood lookingPowder deliveryAntiviralsSolubilityMedical prescription
The invention relates to an acyclovir lyophilized formulation for injection and a preparation method of the acyclovir lyophilized formulation. The acyclovir lyophilized formulation for injection comprises acyclovir, sodium hydroxide, mannitol and a pH value stabilizing agent, wherein the weight ratio of the acyclovir to the mannitol is 1;(1-10). The acyclovir lyophilized formulation is advanced in prescription and good in product formability; the solution before being frozen is clear in appearance; the lyophilized product is good in solubility; and the re-dissolved solution is good in clarity, low in impurity content, good in stability and controllable in quality.
Owner:湖北潜龙药业有限公司
Method for preparing lipidosome coated with protein drugs
ActiveCN104083326AEffective control of particle sizeUniform particle sizePeptide/protein ingredientsGenetic material ingredientsLipid formationOrganic solvent
The invention relates to a novel method for preparing small-particle-size high-encapsulation-efficiency lipidosome coated with protein drugs and belongs to the technical field of biological medicines. The preparation process comprises the following steps: a, dissolving lipids for forming the lipidosome in an organic solvent, mixing the lipids, a protein solution and a proper amount of surfactant according to a proper ratio, and preparing a micro-emulsion phase; b, dissolving the lipids for forming the lipidosome in an organic solvent, mixing and hydrating by using water or a buffer solution or alcohol according to a proper ratio, and preparing a micellar phase; and c, mixing the micro-emulsion phase and the micellar phase, reversing the phase b into a reverse micelle, reversing the phase b again in the process of removing the organic solvent under reduced pressure, finally covering the surface of the phase a micelle with the reverse micelle, and forming the lipidosome. The encapsulated protein drug lipidosome has the average particle size of about 80nm, and the encapsulation ratio can be over 90 percent. The preparation process is simple in process, the production cost is reduced, and industrial production is easily realized.
Owner:SHENYANG PHARMA UNIVERSITY
Populus diversifoli schrenk alkaline soda water
InactiveCN107744081AImprove nutritional qualityImprove nutrientsNatural extract food ingredientsFood ingredient functionsNutrientPeppermints
The invention relates to populus diversifoli schrenk alkaline soda water. The populus diversifoli schrenk alkaline soda water has the advantages that after a populus diversifoli schrenk alkaline extracting solution is added into pure water, two or three of citric acid, honey and peppermint essence are added, so as to prepare the populus diversifoli schrenk alkaline soda water with various tastes;the natural populus diversifoli schrenk alkaline extracting solution is used for replacing the added sodium bicarbonate, trace elements, vitamins and the like in the traditional soda water, so that the nutrient quality of the soda water is improved; the thirst is relieved, the acid and alkaline degree in the human body is balanced, the poor digestion and constipation are relieved, the effects of beautifying and nourishing face are realized, the uric acid is favorably excreted, and then the function of preventing and controlling hyperuricemia or gout is realized; the problems of single mouth feel and lack of nutrients in the soda water are effectively relieved, the consumption tendency of people is met, and the nutritional matters required by the human body are supplemented; the technologyis simple, and the nutritional matters are further remained.
Owner:吐尔逊托乎提
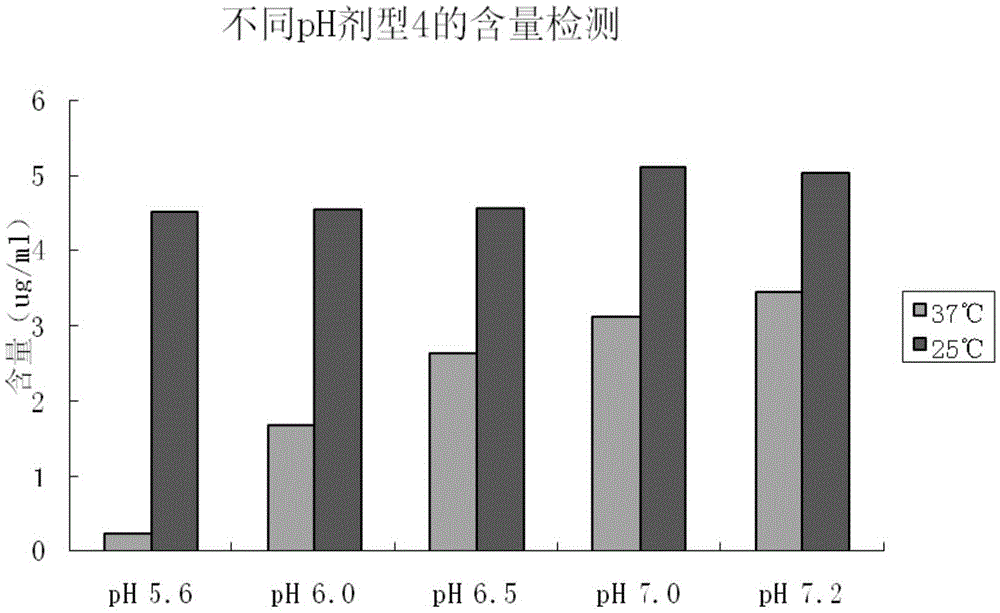
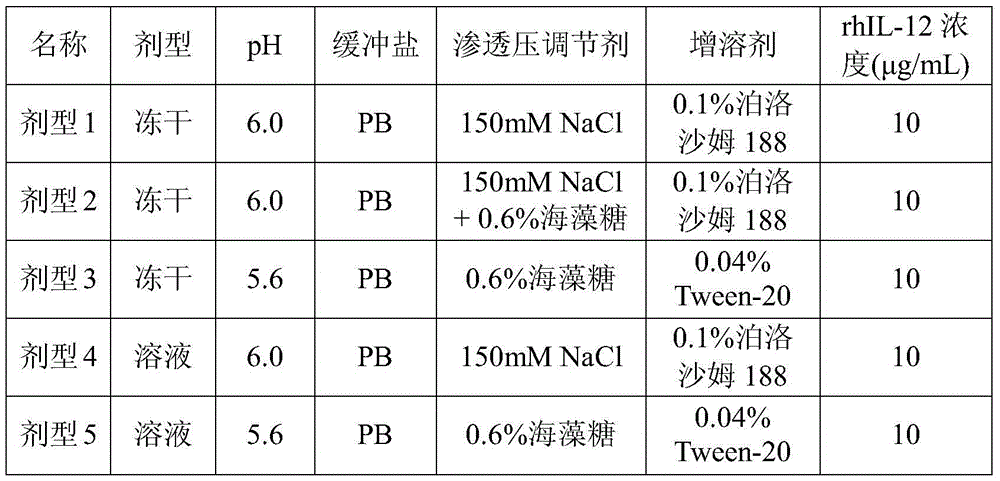
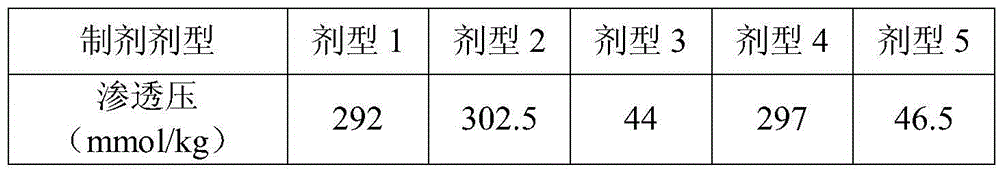
Solution agent for recombining human interleukin 12 and preparation method thereof
ActiveCN105412909ADoes not reduce protein contentDoes not reduce biological activityPeptide/protein ingredientsDigestive systemPreservative freeBlood component
The invention provides a solution agent for recombining human interleukin 12 and a preparation method thereof. The solution agent comprises the human interleukin 12, a phosphate buffer (PB), NaCl and poloxamer 188, wherein pH of the solution agent is maintained to range from 6.0 to 7.2 through the phosphate buffer. The solution agent is good in stability, clear in appearance and free of insoluble substances, preservative and blood components, and the in-vitro activity of contained active polypeptide is stable; under specified conditions, the solution agent can be stably preserved for at least eight months, and compared with a powder injection, the production quality is obviously improved.
Owner:KANGLITAI BIOMEDICAL (QINGDAO) CO LTD
Preparation method of pitaya juice
PendingCN110881594ABright colorDelicate and refreshing tasteFood ingredient as antioxidantFood ingredient as colourBiotechnologyFruit juice
The invention, which relates to the technical field of beverage processing, provides a preparation method of pitaya juice. The preparation method of the pitaya juice comprises the following steps: (1), carrying out pitaya pretreatment; (2), carrying out peel treatment; (3) carrying out pulp treatment; (4), carrying out juice blending; (5), carrying out juice treatment; and (6), carrying out filling and sterilization. The pectin and haematochrome in peels are extracted as additives to prepare pitaya juice, so that waste is turned into wealth and the comprehensive utilization rate of pitaya is greatly increased; and the prepared pitaya juice is clear and bright in color and luster, refreshing and fine in taste, uniform and stable in juice state and harmonious and rich in aroma.
Owner:GUANGXI ZHUANG AUTONOMOUS REGION ACAD OF AGRI SCI
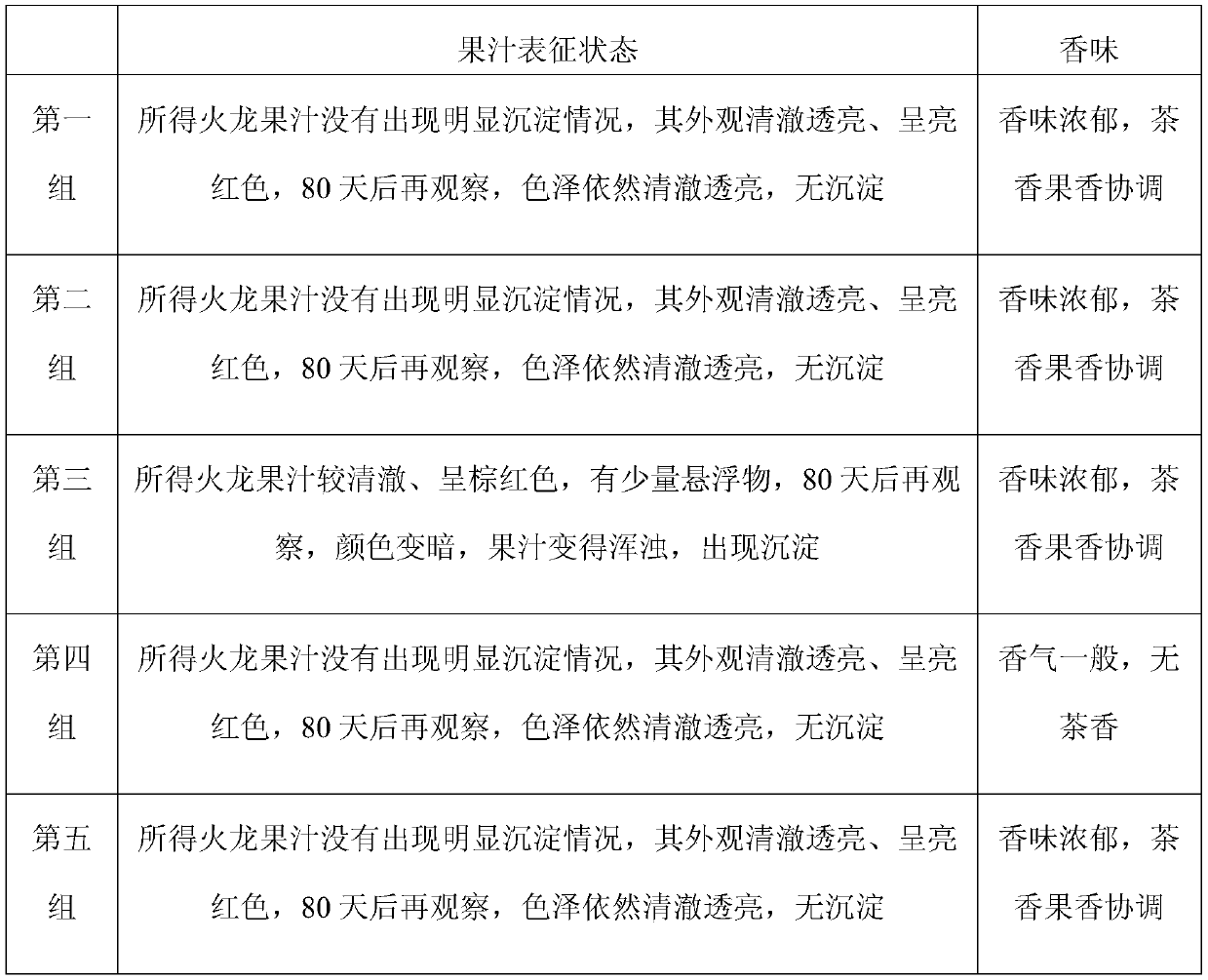
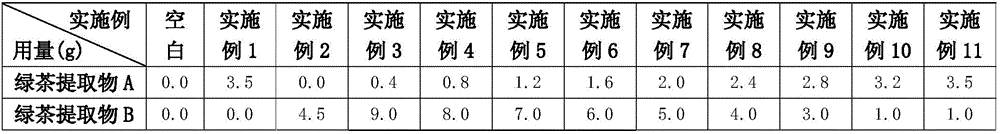
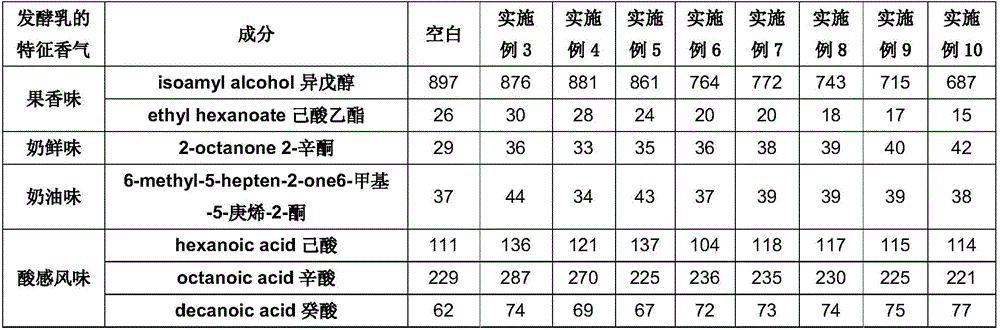
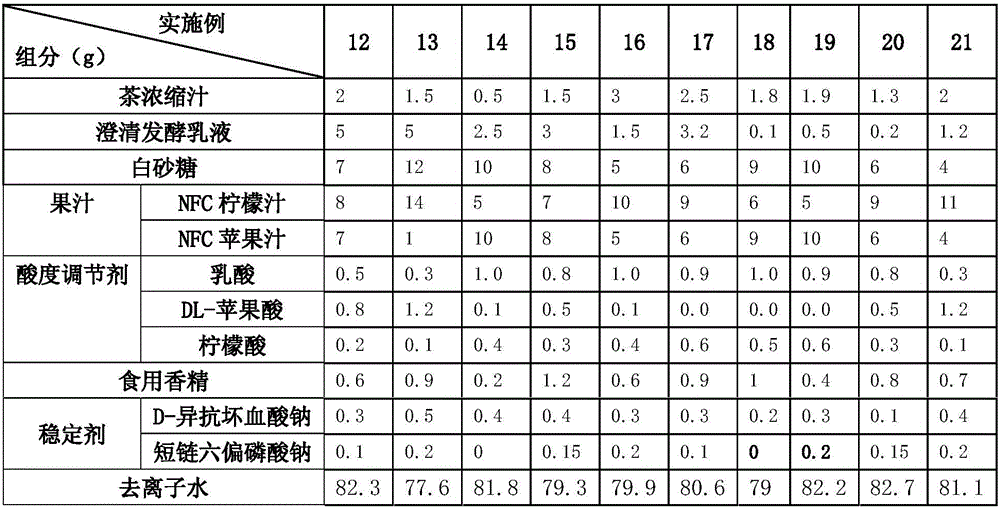
Lactic acid bacteria flavor tea beverage and preparation method thereof
ActiveCN106212804AAdd flavorRefreshing tasteTea extractionClimate change adaptationLactobacillusFlavor
The invention discloses a lactic acid bacteria flavor tea beverage and a preparation method thereof. The lactic acid bacteria flavor tea beverage contains the following ingredients by weight: 0.5%-3% of tea juice, 7%-12% of white sugar, 0.1%-5% of clarified fermented emulsion, 0.1%-1.5% of an acidity regulator, 0.1%-15% of juice, 0.1%-1.2% of edible flavor, 0.15%-0.85% of a stabilizer, and the balance of deionized water. The clarified fermented emulsion is obtained by mixing with a green tea extract and extracting. The preparation method not only can keep the flavor of the lactic acid bacteria, but also can reflect the refreshing taste of the tea beverage; and the beverage have relatively clarified appearance, improved stability and good flavor.
Owner:UNI PRESIDENT ENTERPRISES CHINA INVESTMENT CO LTD KUNSHAN RES & DEV CENT +1
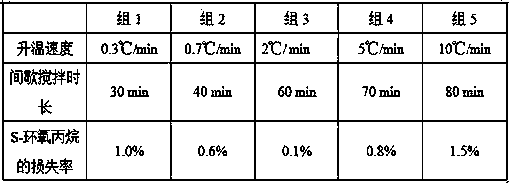
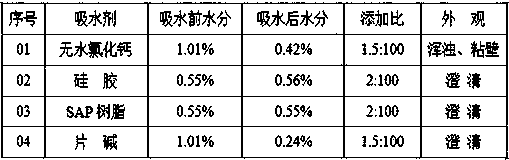
Method for reducing moisture content of byproduct S-epoxy propane in production of R-propylene carbonate
InactiveCN108276362AReduce moistureAppearance clarificationOrganic chemistrySuper absorbentChemistry
The invention provides a method for reducing the moisture content of byproduct S-epoxy propane in production of R-propylene carbonate. The method comprises the following steps: adding caustic soda flakes into a distillation kettle; adding the byproduct S-epoxy propane in the production of the R-propylene carbonate; starting and stirring; then distilling and cooling and recycling a fraction, i.e.,the byproduct S-epoxy propane with the reduced moisture content. According to the method provided by the invention, the moisture content of the byproduct S-epoxy propane in the production of the R-propylene carbonate is reduced to 0.24 to 0.34 percent and the product appearance keeps clear; compared with other methods for reducing moisture through high-tower-plate distillation, distillation afterwater absorption of anhydrous calcium chloride, silica gel and SAP (Super Absorbent Polymer) resin and the like, the method has the characteristics of low equipment investment, small dosage, good effect, low cost, easiness for washing, low energy consumption, easiness for controlling, stability in production, environment friendliness and the like and is suitable for industrial production.
Owner:WEIFANG HUITAO CHEM
Method for preparing ground sesameseed oil
InactiveCN104194922AReduce moistureLow impurity contentFatty-oils/fats refiningFatty-oils/fats productionMoistureBiology
The invention provides a method for preparing ground sesameseed oil with low content of moisture and impurities. The method for preparing the ground sesameseed oil comprises the following steps: firstly, screening, namely purchasing high-quality sesame seeds, eliminating impurities, such as soil, gravel and scrap ion, and weed seeds and immature sesame seeds in the sesame seeds manually or by adopting a raw material cleaning car; secondly, parching, namely parching the screened sesame seeds by adopting a roller parching machine while temperature is maintained to be 160 DEG C for ten minutes and then the temperature is maintained to be 110 DEG C; thirdly, carrying out cold pressing, namely introducing the parched sesame seeds into a raw material hopper of a presser, and pressing oil; fourthly, adding a strong alkali and weak acid salt solution with pH value of 7-9 into the pressed oil, so that hydrated pressed oil is obtained; fifthly, pumping the hydrated pressed oil into a bag filtering machine by virtue of a pipeline pump, filtering out impurities, and directly introducing clear oil obtained after filtering to a finished oil product tank.
Owner:SHUYANG SU HE AGRI PROD SALES PROFESSIONAL COOP ASSOC
Lornoxicam freeze-dried injection and preparation method thereof
ActiveCN100560061CHigh clarityGood formabilityPowder deliveryOrganic active ingredientsMedicineLornoxicam
Owner:HAINAN JINRUI PHARMA CO LTD
Dextrooracetam freeze-dried powder injection with good stability and preparation method thereof
InactiveCN110327301AImprove stabilityHigh clarityPowder deliveryOrganic active ingredientsFreeze-dryingMoisture
The invention provides a dextrooracetam freeze-dried powder injection with good stability; the dextrooracetam freeze-dried powder injection is prepared by freeze-drying a solution prepared from crystalline-form dextrooracetam, a freeze-dried excipient, a metal ion chelating agent, a pH regulator and water for injection. The obtained freeze-dried powder injection sample has no obvious change in properties, related substances and content under the conditions of high humidity and high temperature through the investigation of influencing factors under the conditions of commercially available packaging, so as to indicate that the freeze-dried powder injection is relatively stable and can effectively prevent moisture. The preparation method is simple and suitable for industrial mass production.
Owner:CHONGQING RUNZE PHARM CO LTD
Progesterone injection and method for preparing the same
InactiveCN101152186BEasy to prepareSmall and uniform particle sizeOrganic active ingredientsEmulsion deliveryProgesteronesPoor quality
The invention provides a progesterone injection and the preparation method, which solves the problems of strong pungency, inconvenient use with prior progesterone injection, the problems of low stability and poor quality with prior progesterone micoremulsion, and the problems of high cost with asepsis preparation process. The weight percentage of the progesterone injection is: 0.5 to 2 percent ofprogesterone, 15 to 35 percent of oil for injection, 1 to 12 percent of emulsifier, 0.1 to 1 percent of assistant emulsifier, 0.2 to 2 percent of stabilizer, 0.01 to 2.5 percent of zntioxidant and allowance of water.
Owner:曲靖平和安康医药保健有限公司
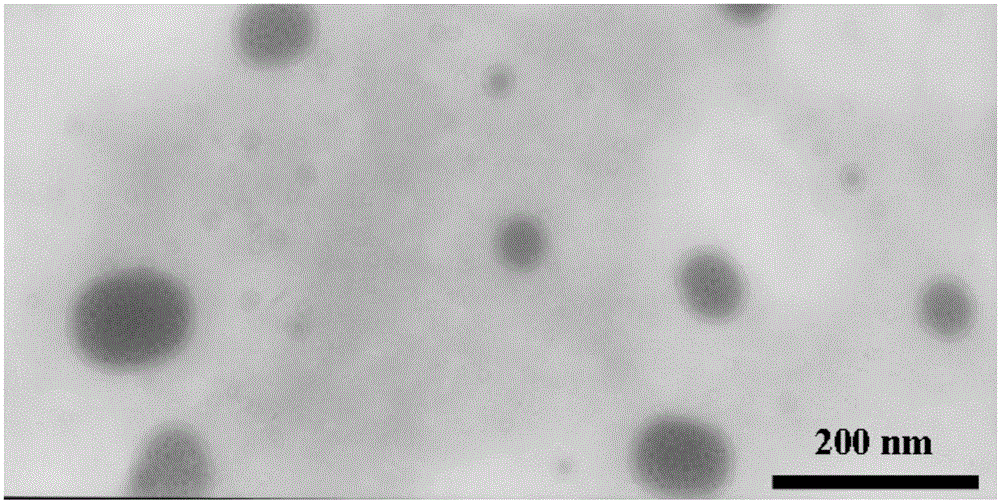
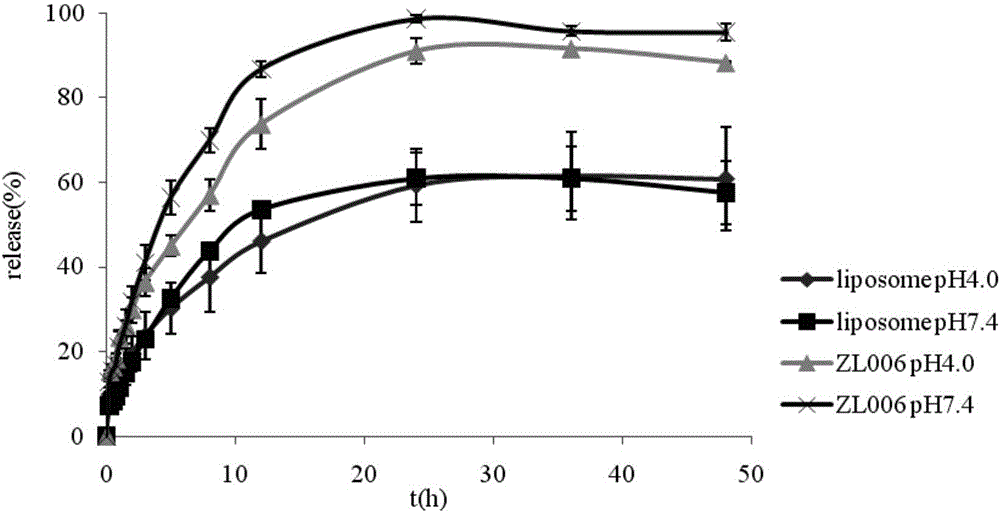
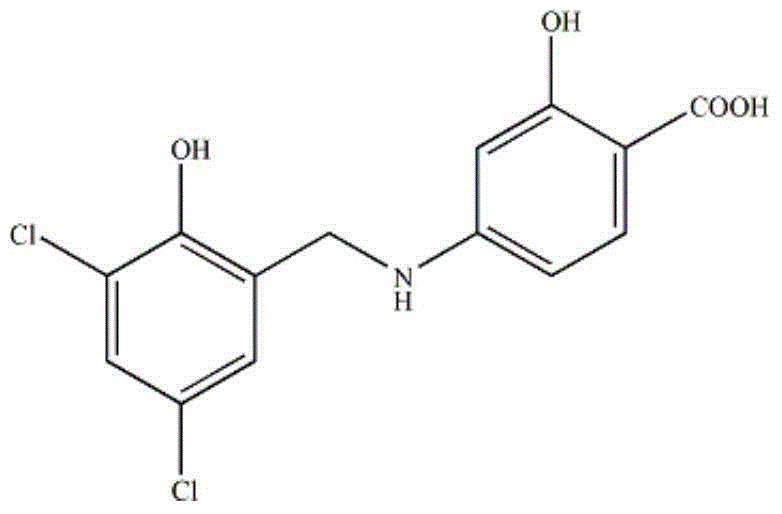
A kind of zl006 liposome and preparation method thereof
InactiveCN103494773BAppearance clarificationQuality improvementOrganic active ingredientsPharmaceutical non-active ingredientsLipid formationOrganic solvent
The invention relates to a ZL006 liposome and a preparation method thereof. The liposome consists of a lipid bilayer and ZL006, wherein the weight ratio of the lipid material of the lipid bilayer to the ZL006 is (10-4):1. The ZL006 liposome can be prepared by a pouring method or a film dispersion method. The preparation using the pouring method comprises the following steps: dissolving the ZL006 and the lipid material into an organic solvent, and uniformly mixing to obtain an organic phase; adding the organic phase into a water phase, reducing the particle size and removing the organic solvent; and filtering through microfiltration membranes with different pore diameters to obtain the ZL006 liposome. According to the ZL006 liposome and the preparation method thereof provided by the invention, by selecting proper material composition and proper preparation technology, the ZL006 liposome with good quality is obtained, the particle size of the liposome is small, the encapsulation efficiency is high, and the stability is good.
Owner:NANJING MEDICAL UNIV
Vidarabine monophosphate freeze-dried powder injection and preparation method thereof
ActiveCN102379853BGood formabilityHigh clarityOrganic active ingredientsPowder deliveryFreeze-dryingPhosphoric acid
The invention relates to a vidarabine monophosphate freeze-dried powder injection and a preparation method thereof, and the vidarabine monophosphate freeze-dried powder injection is prepared by freeze-drying vidarabine monophosphate, sodium hydroxide solution and water for injection, wherein the sodium hydroxide solution is used as a pH regulator, and the sodium hydroxide solution is used until the pH value of liquid medicine is 7.0-7.5. The vidarabine monophosphate freeze-dried powder injection provided by the invention is few in types of auxiliary materials, low in using amount, good in forming property of a freeze-dried product, good in re-dissolving property, clear in appearance of the solution before freezing, good in clarity after being freeze-dried, low in content of impurities, good in stability and controllable in quality, and can reduce the potential safety hazard of the vidarabine monophosphate freeze-dried powder injection, and improve the efficacy of the vidarabine monophosphate freeze-dried powder injection.
Owner:HAINAN JINRUI PHARMA CO LTD
Anti-foaming agent special for aluminum oxide plant and preparation method of anti-foaming agent
ActiveCN109621497AAppearance clarificationLow viscosityFoam dispersion/preventionFoaming agentPolypropylene glycol
The invention relates to the technical field of aluminum oxide production, in particular to an anti-foaming agent special for an aluminum oxide plant and a preparation method of the anti-foaming agent. The anti-foaming agent special for the aluminum oxide plant is prepared from, by mass, 25-30 parts of base oil, 30-35 parts of polypropylene glycol, 20-25 parts of iso-tridecanol polyoxyethylene ether, 15-20 parts of polyoxyethylene lauryl ether, 5-10 parts of Tween 80 and 2-3 parts of sodium hydroxide. The anti-foaming agent has an excellent eliminating effect on foam generated by aluminum hydroxide refined liquid, and has a good foam inhibition effect, the stability of the product is high, the agent adding amount is low, the performance of the anti-foaming agent is similar to that of the foreign Nalco similar products, but the price is lower than that of the similar products, and the cost performance is more outstanding. The invention further provides a preparation method of the anti-foaming agent.
Owner:邢国艾
Dantrolene sodium freeze-dried powder injection for injection and preparation method thereof
ActiveCN102302461BGood formabilityHigh clarityOrganic active ingredientsPowder deliveryForeign matterCLARITY
The invention belongs to the technical field of medicines, and particularly relates to a dantrolene sodium freeze-dried powder injection for injection and a preparation method thereof. The freeze-dried powder injection is prepared by freeze drying dantrolene sodium, lactobiose, a pH regulator and water for injection, wherein a mass ratio of the dantrolene sodium to the lactobiose is (1:0.5) to (1:2.5). The preparation method comprises the following steps of: adding the dantrolene sodium to the lactobiose in a prescription amount into the water for the injection, stirring, regulating the pH value to 9.0 to 10.5 by using the pH regulator, adding active carbon for injection, removing a heat source, decolorizing, filtering for decarburizing, performing fine filtering by using a filter membrane, packaging in separate bags, freezing and drying. The dantrolene sodium freeze-dried powder injection is studied on the basis of a freeze-dried process, namely the dantrolene sodium freeze-dried powder injection is cooled, heated by a small margin and is cooled again, so that the moisture of the freeze-dried product is reduced, and the freeze-dried product is high in redissolution and visible foreign matters and does not have insoluble granules. The dantrolene sodium freeze-dried powder injection for the injection is high in resolubility, clarity and stability, and low impurity content.
Owner:HAINAN JINRUI PHARMA CO LTD
Stabilizer and stabilizing method for honeysuckle extracted solution
ActiveCN105233295AProduct content is stableAppearance remains clarifiedMacromolecular non-active ingredientsPlant ingredientsChemistryMaltodextrin
The invention discloses a stabilizer and a stabilizing method for a honeysuckle extracted solution, belongs to the technical field of pharmacy, and relates to extraction of medicinal materials, namely, honeysuckle. The problems that an existing honeysuckle extracted solution is instable in storage and turbid in appearance, the content of chlorogenic acid is gradually reduced, and PH is changed are solved. The stabilizer for the honeysuckle extracted solution is prepared from pea maltodextrin, beta-cyclodextrin and / or hydroxypropyl-beta-cyclodextrin. Cyclodextrin and pea maltodextrin are mixed to serve as the stabilizer, the advantages of being high in solubility and solubilization of cyclodextrin cavities and pea maltodextrin are combined, the stabilizer is mixed with the honeysuckle extracted solution according to a certain proportion, it can be guaranteed that the PH value of the solution is basically unchanged, the product content is stable, and the appearance is kept clear.
Owner:王志敏
Application of pazopanib, pharmaceutical composition, injection, preparation method and application
PendingCN113350351ANo accumulationImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismDiseaseCyclodextrin
The invention discloses application of pazopanib, a pharmaceutical composition, an injection, a preparation method and application. The pharmaceutical composition comprises the following components: pazopanib, a cyclodextrin solubilizer and a solvent, wherein the mass ratio of pazopanib to the cyclodextrin solubilizer is 1: (13-100); and based on 1mL of the pharmaceutical composition, the concentration of the cyclodextrin solubilizer in the pharmaceutical composition is 6-330mg / mL. The pharmaceutical composition prepared by the invention can be used for treating diseases such as acute lung injury, pulmonary fibrosis and acute respiratory distress syndrome, and the treatment aim can be achieved by using a small amount of pazopanib; and moreover, the pharmaceutical composition is high in bioavailability, high in stability and low in impurity content, and the phenomenon of drug accumulation is avoided.
Owner:青晓制药公司
Globulin pasteurization process using combined protective agent
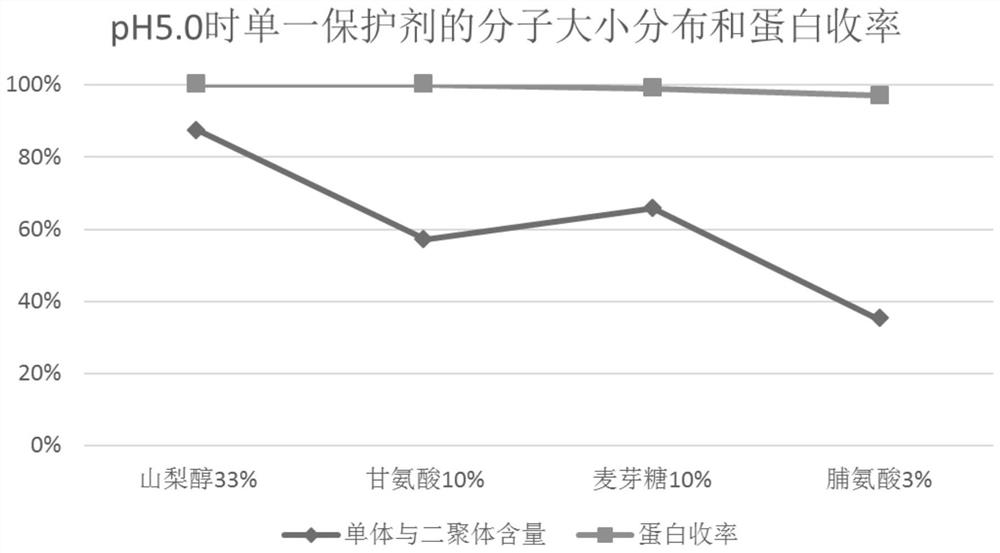
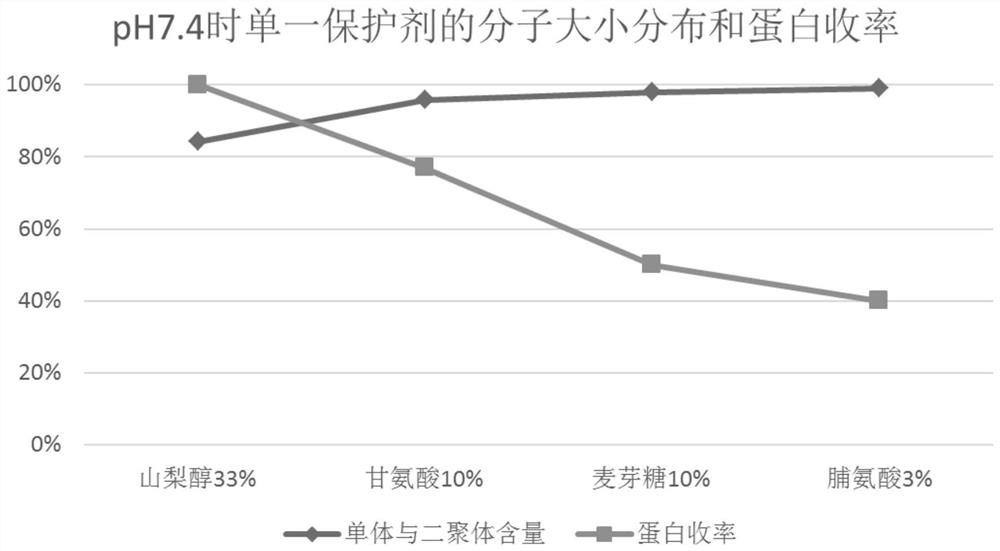
PendingCN114099721AAppearance clarificationReduce process troubleHeatHuman immune globulinMolecular biology
The invention relates to the technical field of human immune globulin inactivation, in particular to a globulin Pasteur inactivation process using a combined protective agent, a protein solution enriched with immune globulin is prepared with plasma as a raw material, Pasteur inactivation is performed on the protein solution, the protein solution contains sorbitol with the concentration of 300-400 g / l and glycine with the final concentration of 80-120 g / l, the pH value of the protein solution is 6.0-7.5, and the protein concentration is 10-30 g / L. According to the scheme, the technical problem that an existing pasteurization human immune globulin inactivation process is difficult to guarantee the protein yield and quality at the same time can be solved. The scheme can be applied to practical operation of production and preparation of the human immune globulin, is simple and easy to implement and low in cost, and has great popularization and application values, and the protein yield can reach 95% or above.
Owner:HUALAN BIOLOGICAL ENG CHONGQING
Special defoamer for alumina plant and preparation method thereof
ActiveCN109621497BGood compatibilityAppearance clarificationFoam dispersion/preventionAluminium hydroxideDioxyethylene Ether
The invention relates to the technical field of aluminum oxide production, in particular to an anti-foaming agent special for an aluminum oxide plant and a preparation method of the anti-foaming agent. The anti-foaming agent special for the aluminum oxide plant is prepared from, by mass, 25-30 parts of base oil, 30-35 parts of polypropylene glycol, 20-25 parts of iso-tridecanol polyoxyethylene ether, 15-20 parts of polyoxyethylene lauryl ether, 5-10 parts of Tween 80 and 2-3 parts of sodium hydroxide. The anti-foaming agent has an excellent eliminating effect on foam generated by aluminum hydroxide refined liquid, and has a good foam inhibition effect, the stability of the product is high, the agent adding amount is low, the performance of the anti-foaming agent is similar to that of the foreign Nalco similar products, but the price is lower than that of the similar products, and the cost performance is more outstanding. The invention further provides a preparation method of the anti-foaming agent.
Owner:邢国艾
Soda water health-care beverage containing cyclocarya paliurus flavone and preparation method of soda water health-care beverage containing cyclocarya paliurus flavone
PendingCN112106910AAppearance clarificationHas a fragranceFood ingredientsPolyamideSodium bicarbonate
The invention provides a soda water health-care beverage containing cyclocarya paliurus flavone and a preparation method of soda water health-care beverage containing cyclocarya paliurus flavone. Thepreparation method comprises the following steps of S1, performing extracting on cyclocarya paliurus leaf powder twice by a leaching method, combining supernatant, performing concentrating and performing freeze drying, to obtain a cyclocarya paliurus flavone crude extract; S2, enabling the cyclocarya paliurus flavone crude extract to be made into a solution, performing filtering, performing filtering with a polyamide chromatographic column, performing concentrating, and performing freeze drying to obtain cyclocarya paliurus flavone; S3, dissolving sodium bicarbonate in purified water to obtaina sodium bicarbonate aqueous solution for later use; and S4, sterilizing the sodium bicarbonate aqueous solution, mixing the sterilized sodium bicarbonate aqueous solution with the cyclocarya paliurus flavone in a sterile environment, and performing sterile filling. The health-care beverage disclosed by the invention has the efficacy of soda water and the efficacy of reducing blood pressure, andis natural in materials, environment-friendly and healthy.
Owner:NINGBO UNIV
A kind of stabilizer and stabilizing method for extracting solution of honeysuckle
ActiveCN105233295BPH value unchangedStable contentMacromolecular non-active ingredientsPlant ingredientsChlorogenic acidBeta-Cyclodextrins
The invention discloses a stabilizer and a stabilizing method for a honeysuckle extracted solution, belongs to the technical field of pharmacy, and relates to extraction of medicinal materials, namely, honeysuckle. The problems that an existing honeysuckle extracted solution is instable in storage and turbid in appearance, the content of chlorogenic acid is gradually reduced, and PH is changed are solved. The stabilizer for the honeysuckle extracted solution is prepared from pea maltodextrin, beta-cyclodextrin and / or hydroxypropyl-beta-cyclodextrin. Cyclodextrin and pea maltodextrin are mixed to serve as the stabilizer, the advantages of being high in solubility and solubilization of cyclodextrin cavities and pea maltodextrin are combined, the stabilizer is mixed with the honeysuckle extracted solution according to a certain proportion, it can be guaranteed that the PH value of the solution is basically unchanged, the product content is stable, and the appearance is kept clear.
Owner:王志敏
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com