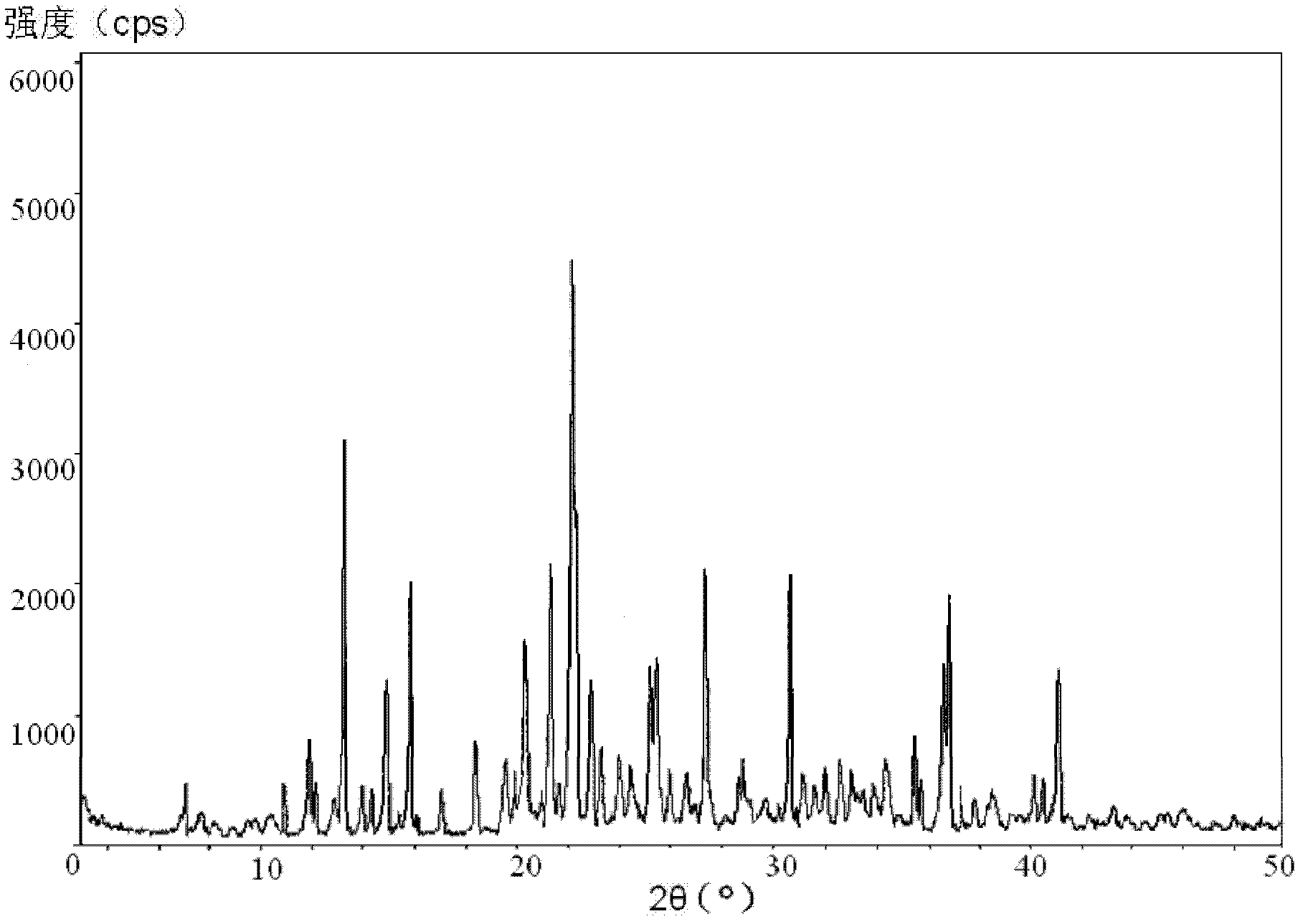
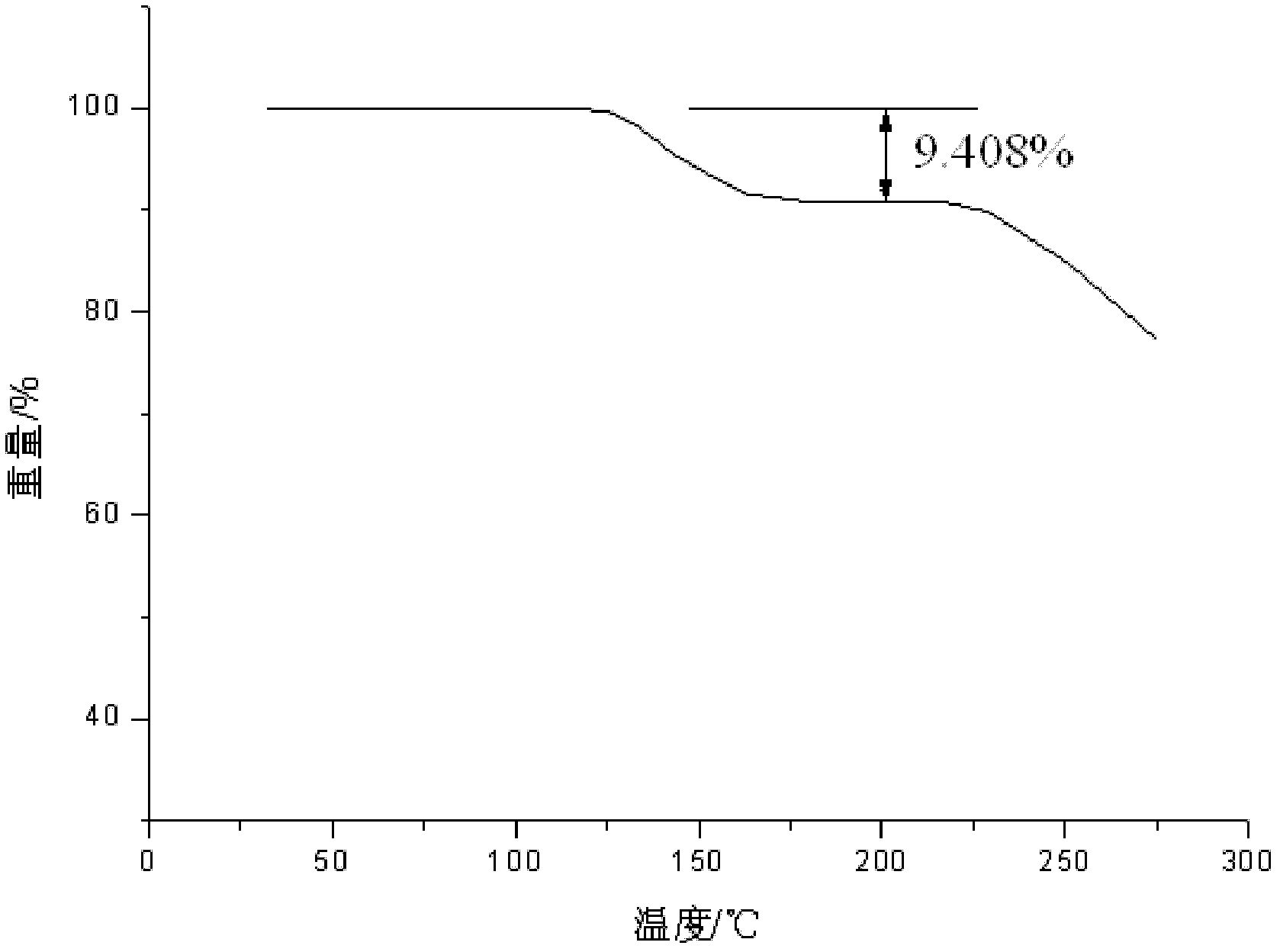
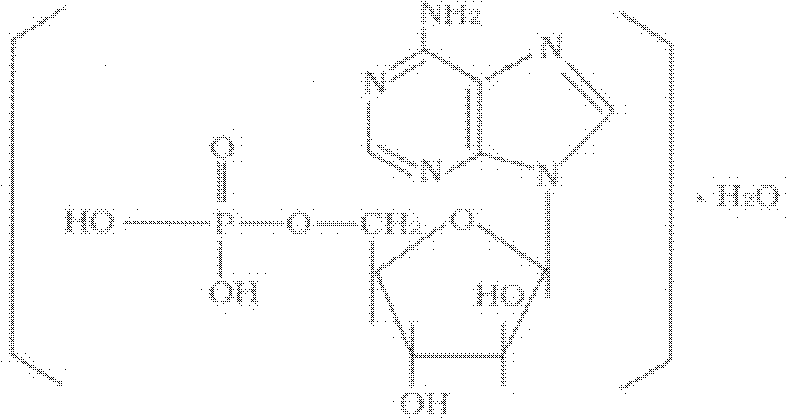
Vidarabine monophosphate freeze-dried powder injection and preparation method thereof
A technology of adenosine vidarabine monophosphate freeze-dried powder and adenosine monophosphate, which is applied in the direction of freeze-drying delivery, antiviral agent, powder delivery, etc., and can solve the problems of reduced product safety, potential safety hazards, and influence on curative effect , to achieve the effects of low scrap rate, reduced production cost, and less types and amounts of auxiliary materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] [Example 1] Preparation of vidarabine monophosphate freeze-dried powder injection
[0057] Prescription (specification 0.1g)
[0058]
[0059]
[0060] Prescription (specification 0.2g)
[0061]
[0062] Preparation Process:
[0063] 1) Cleaning and sterilization of bottles, butyl rubber stoppers and aluminum-plastic combination caps;
[0064] 2) Preparation of medicinal solution: weighing the prescribed amount of adenosine monophosphate raw material. First add adenosine monophosphate raw material drug into the water for injection, slowly add 20% sodium hydroxide solution to a clear solution while stirring, adjust the pH value of the liquid to 7.0, replenish the water for injection to the full amount, add medicinal activated carbon, and circulate Stir, decarbonize, filter, sample, pass the test, filter through a 0.22μm primary sterilizing filter and 0.22μm secondary terminal sterilizing filter, and put it into the liquid medicine barrel for filling;
[0065...
Embodiment 2
[0070] [Example 2] Preparation of vidarabine monophosphate freeze-dried powder injection
[0071] Prescription (specification 0.1g)
[0072]
[0073] Prescription (specification 0.2g)
[0074]
[0075]
[0076] Preparation Process:
[0077] 1) Cleaning and sterilization of bottles, butyl rubber stoppers and aluminum-plastic combination caps;
[0078] 2) Preparation of medicinal solution: weighing the prescribed amount of adenosine monophosphate raw material. First add adenosine monophosphate raw material drug into the water for injection, slowly add 20% sodium hydroxide solution to a clear solution while stirring, adjust the pH value of the liquid to 7.2, replenish the water for injection to the full amount, add medicinal activated carbon, and circulate Stir, decarbonize, filter, sample, pass the test, filter through a 0.22μm primary sterilizing filter and 0.22μm secondary terminal sterilizing filter, and put it into the liquid medicine barrel for filling;
[0079...
Embodiment 3
[0084] [Example 3] Preparation of vidarabine monophosphate freeze-dried powder injection
[0085] Prescription (specification 0.1g)
[0086]
[0087] Prescription (specification 0.2g)
[0088]
[0089] Preparation Process:
[0090] 1) Cleaning and sterilization of bottles, butyl rubber stoppers and aluminum-plastic combination caps;
[0091] 2) Preparation of medicinal solution: weighing the prescribed amount of adenosine monophosphate raw material. First add adenosine monophosphate raw material drug into the water for injection, slowly add 20% sodium hydroxide solution to a clear solution while stirring, adjust the pH value of the liquid to 7.3, replenish the water for injection to the full amount, add medicinal activated carbon, and circulate Stir, decarbonize, filter, sample, pass the test, filter through a 0.22μm primary sterilizing filter and 0.22μm secondary terminal sterilizing filter, and put it into the liquid medicine barrel for filling;
[0092]3) The filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com