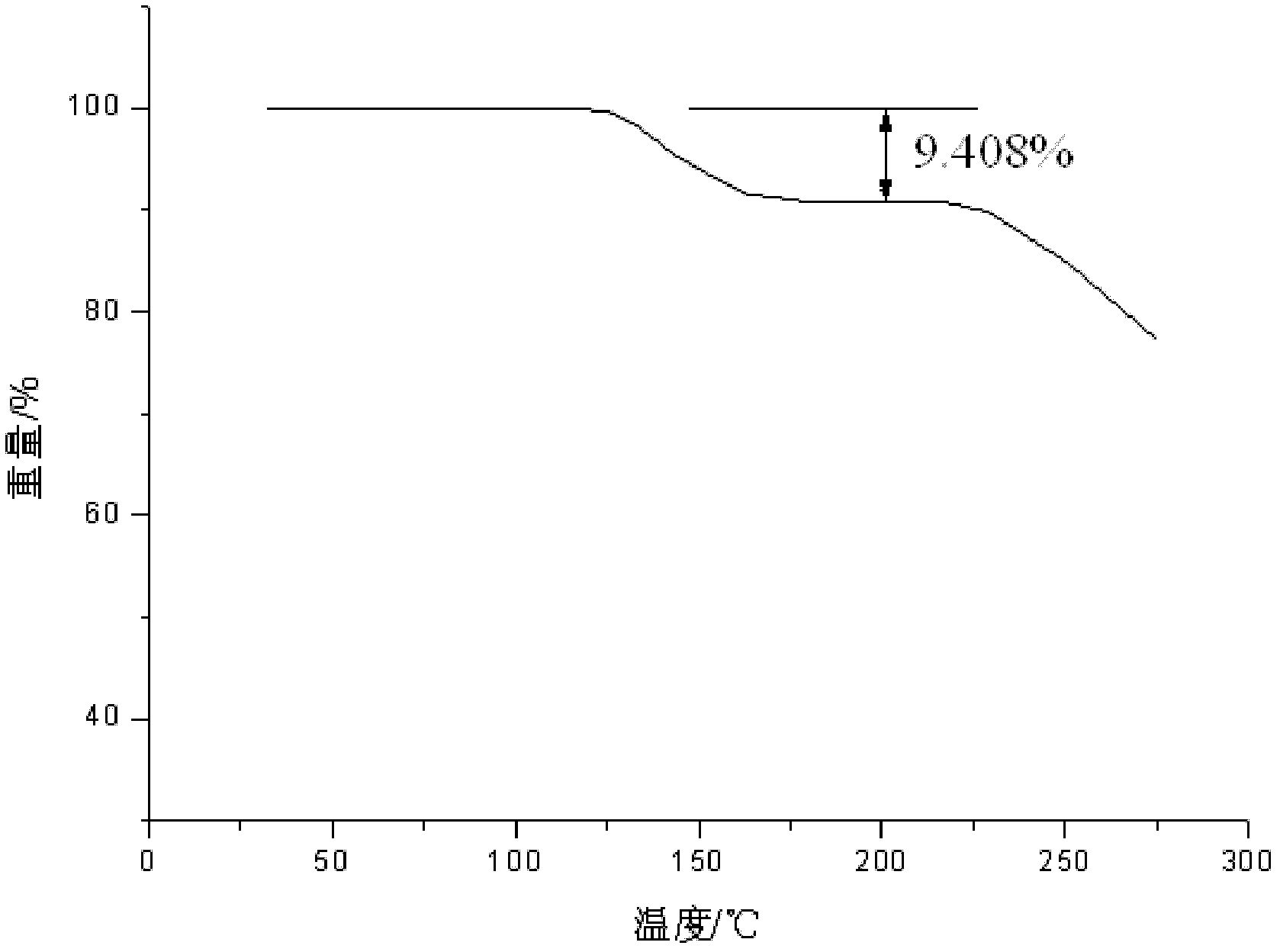
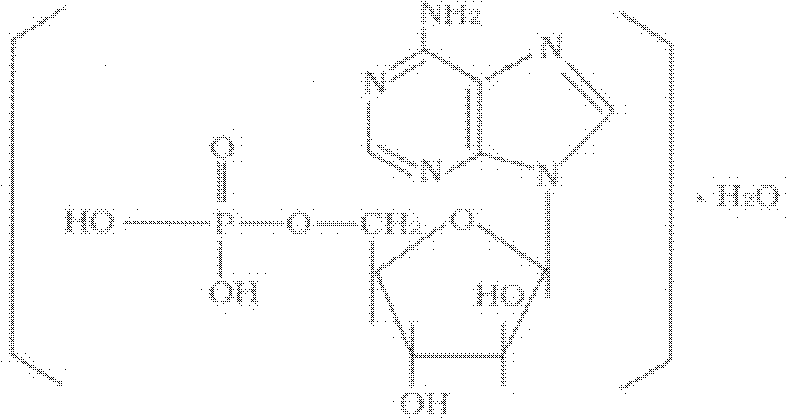
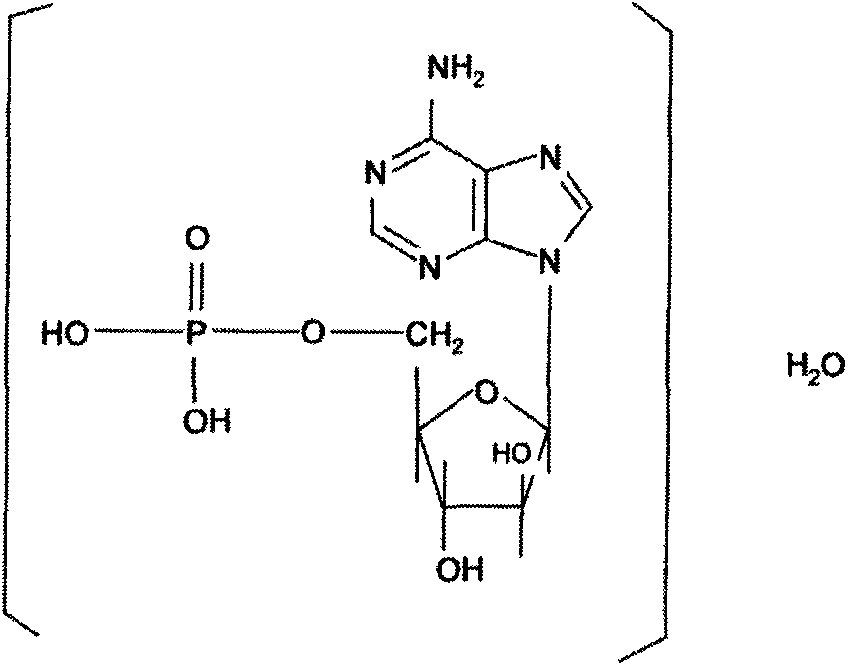
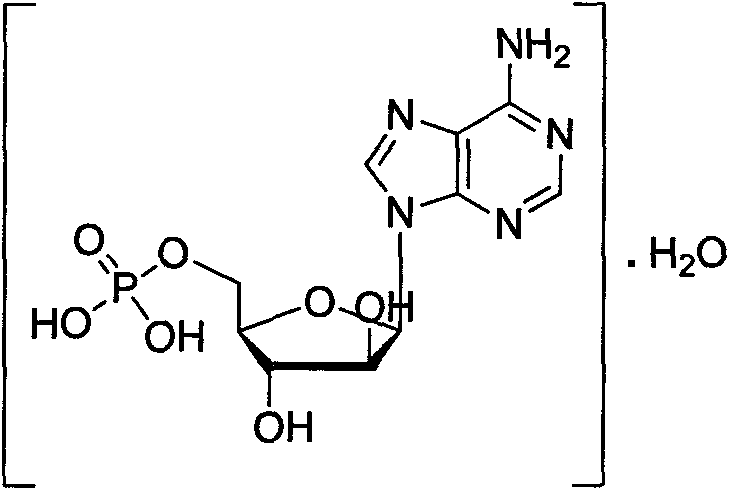
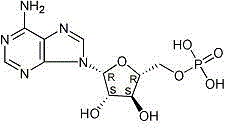
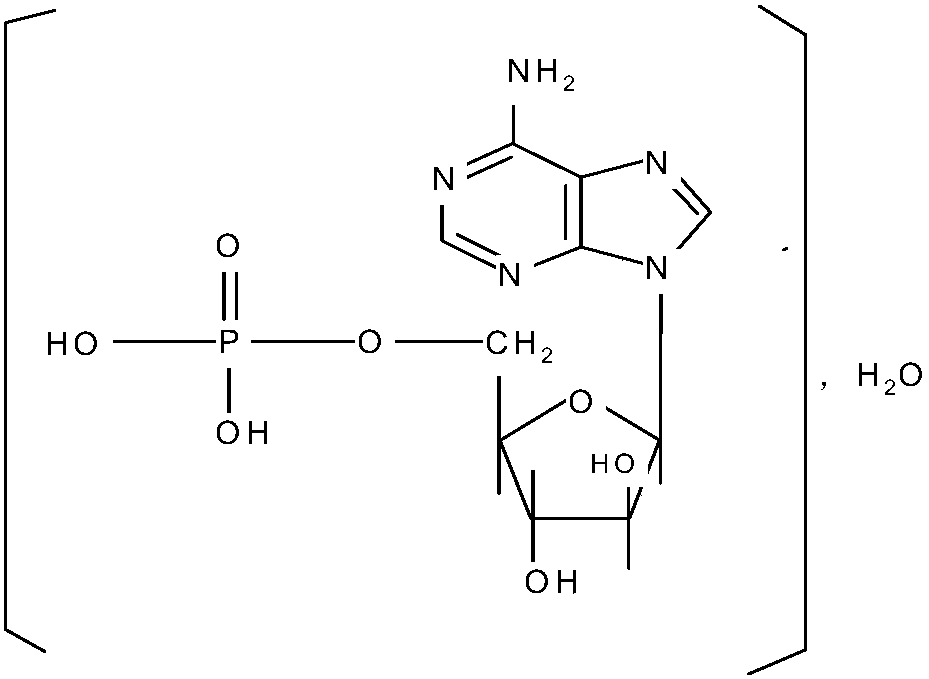
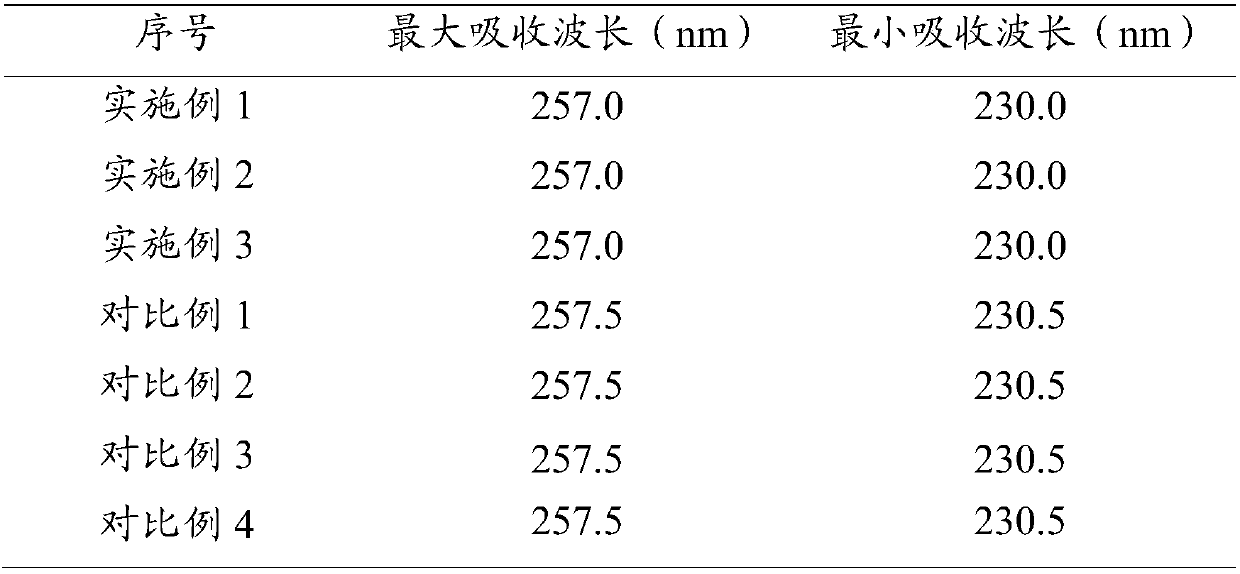
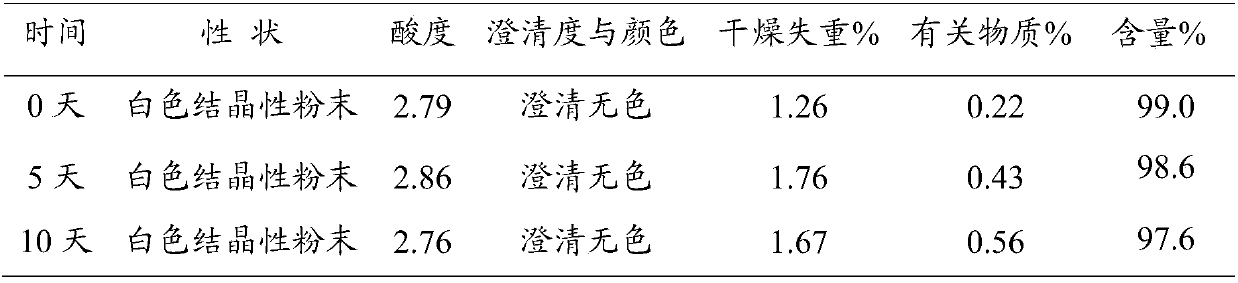
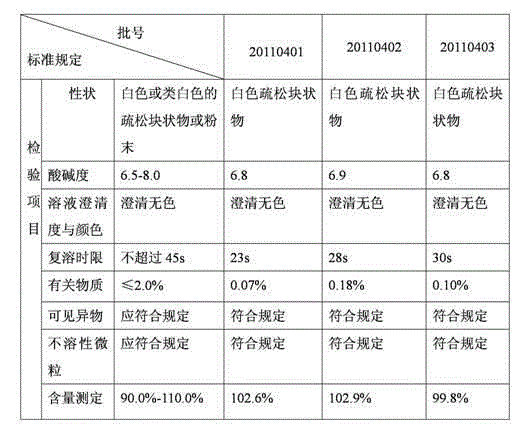
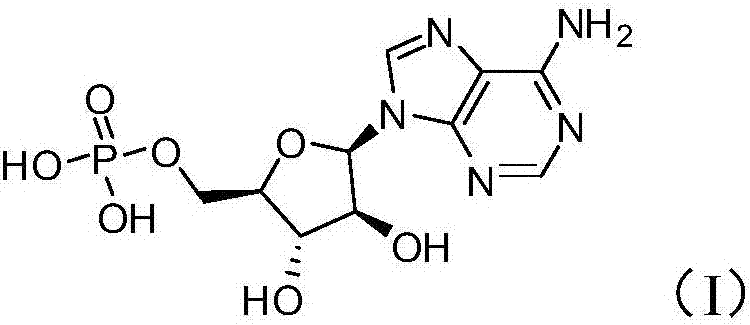
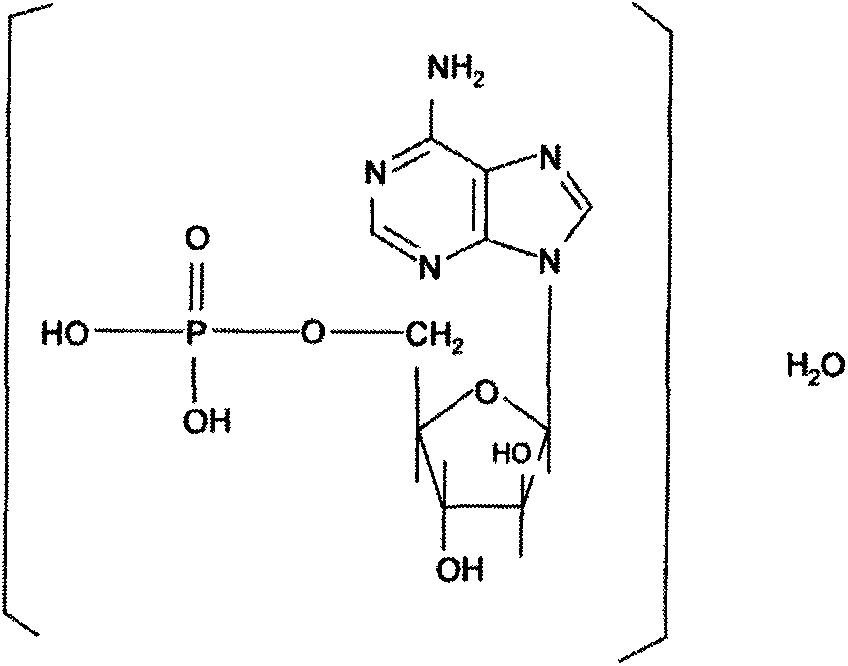
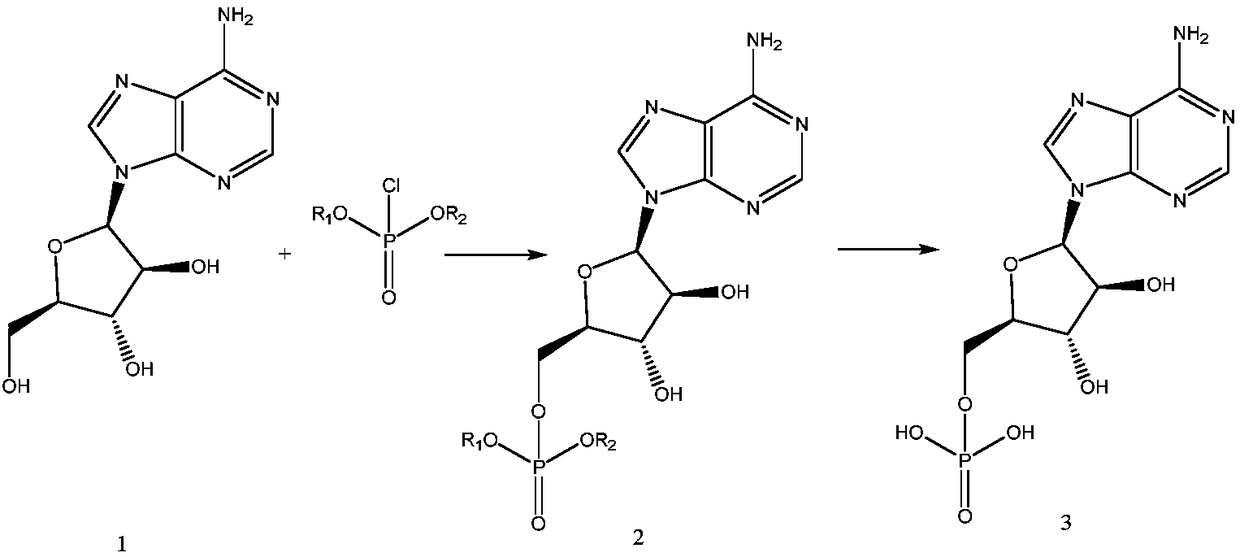
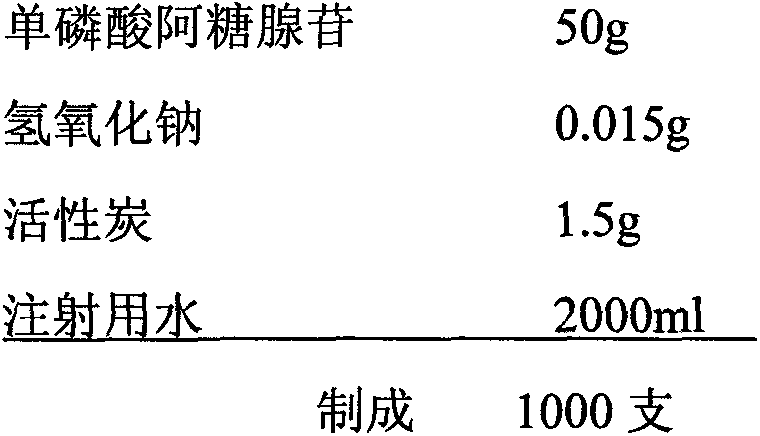
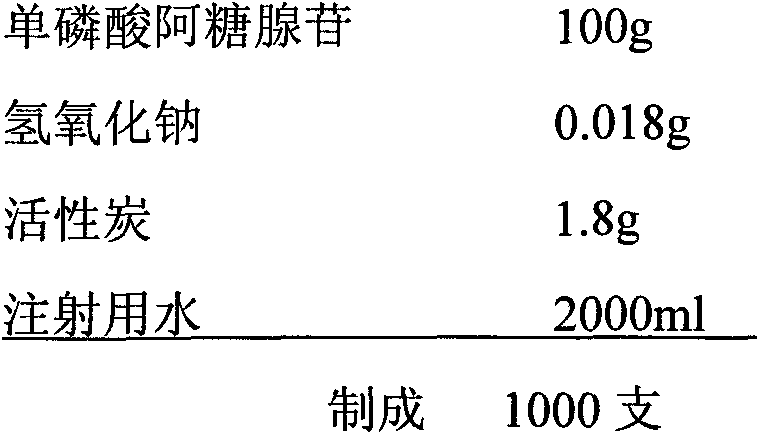
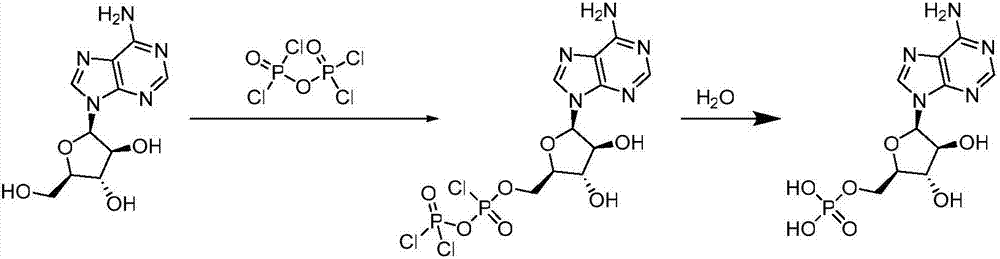
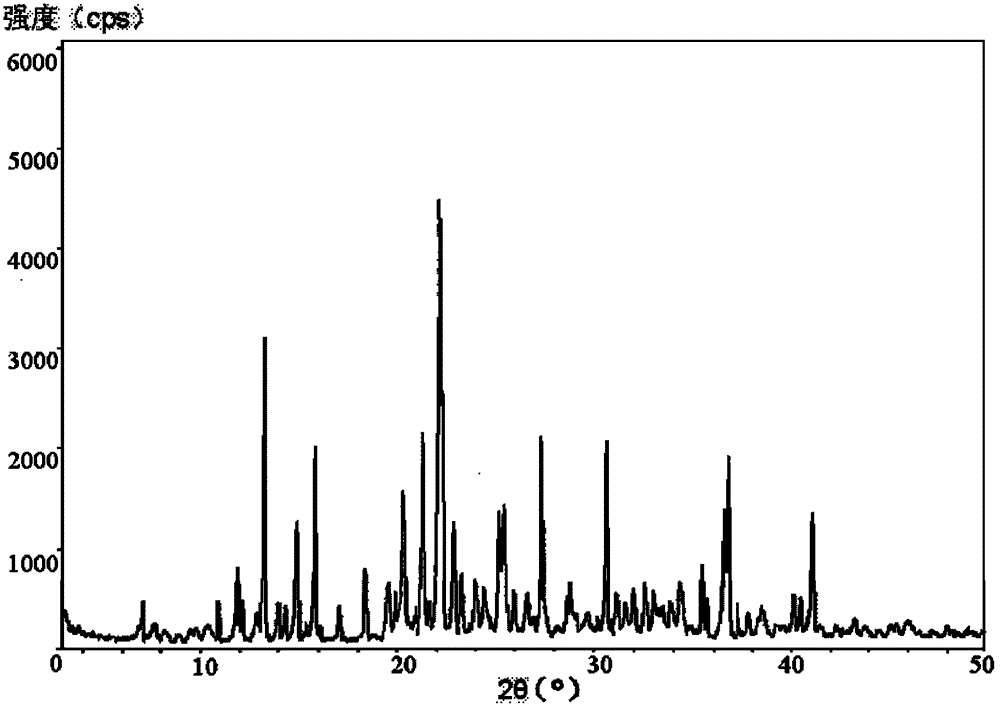
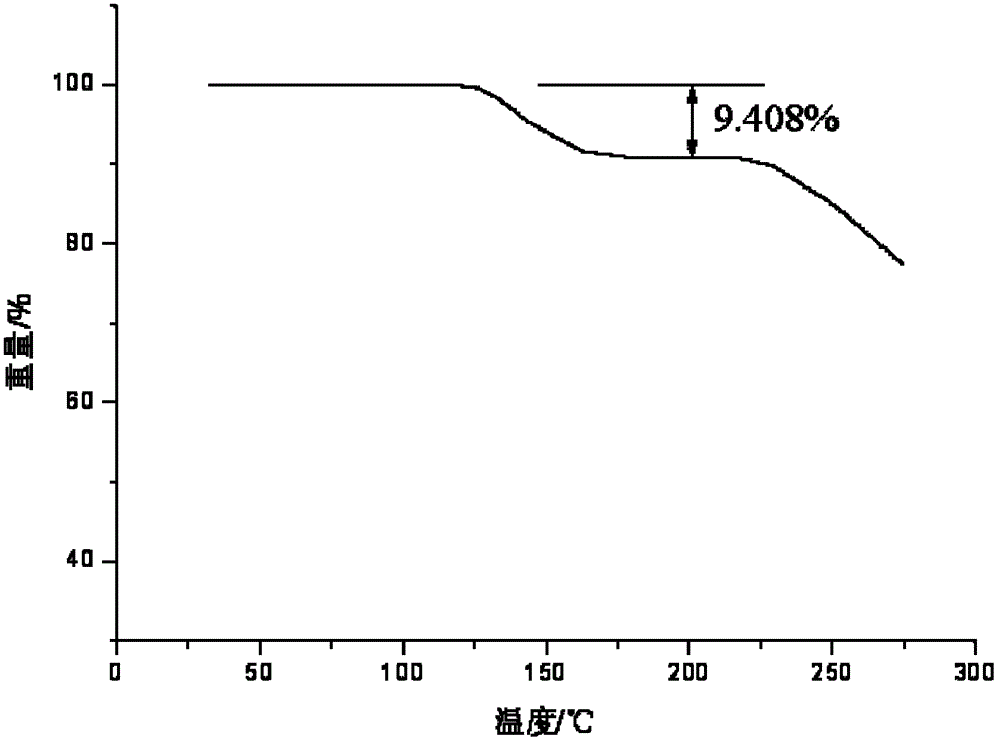
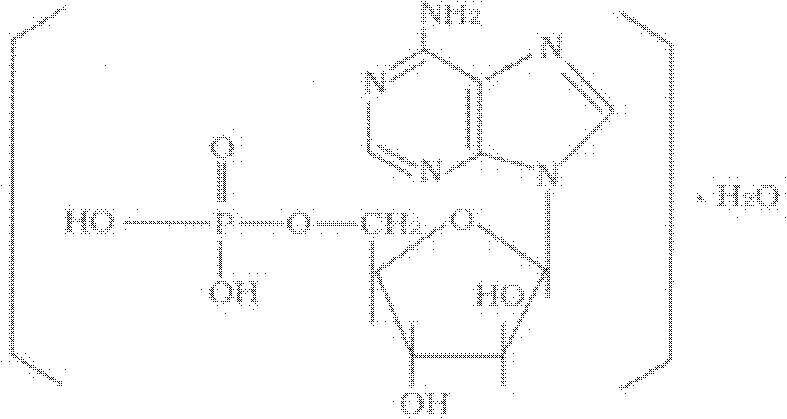
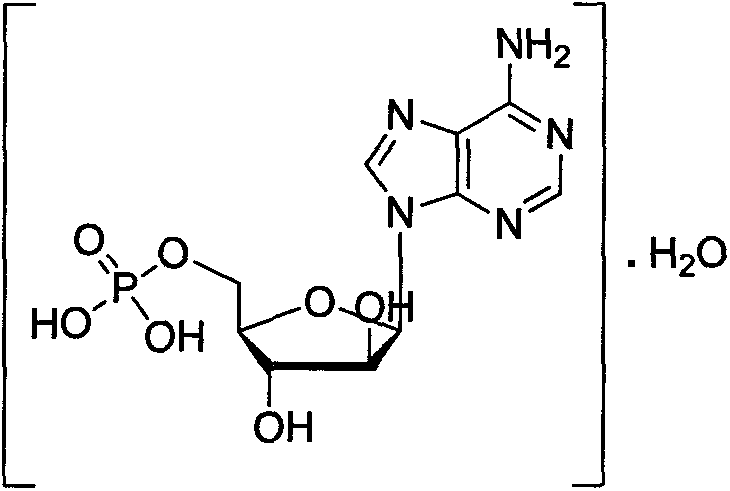
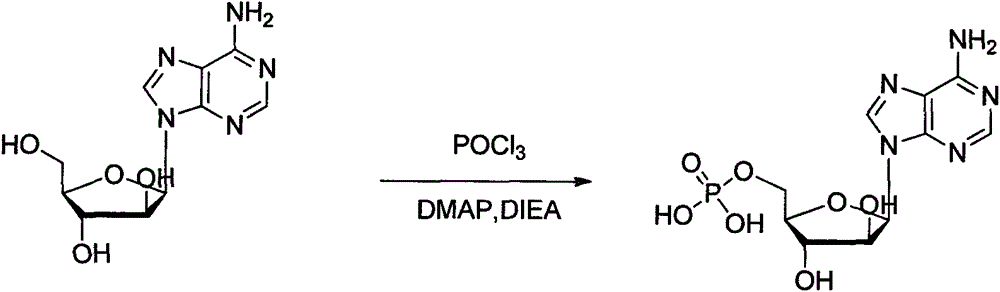
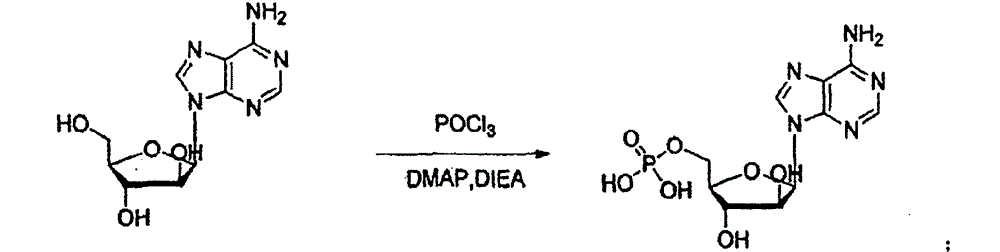
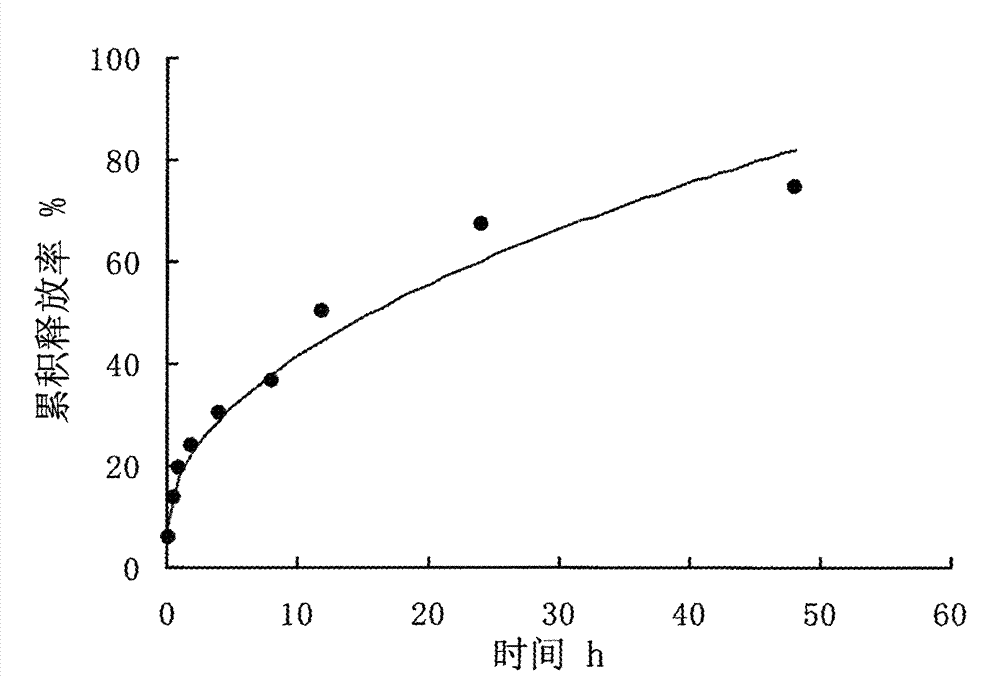
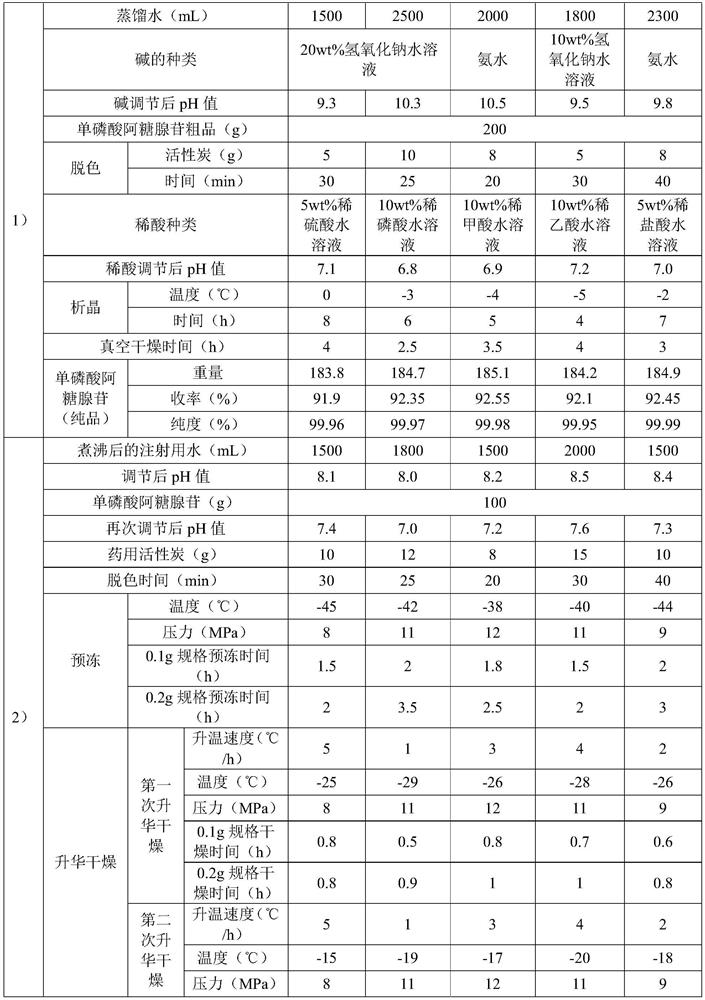
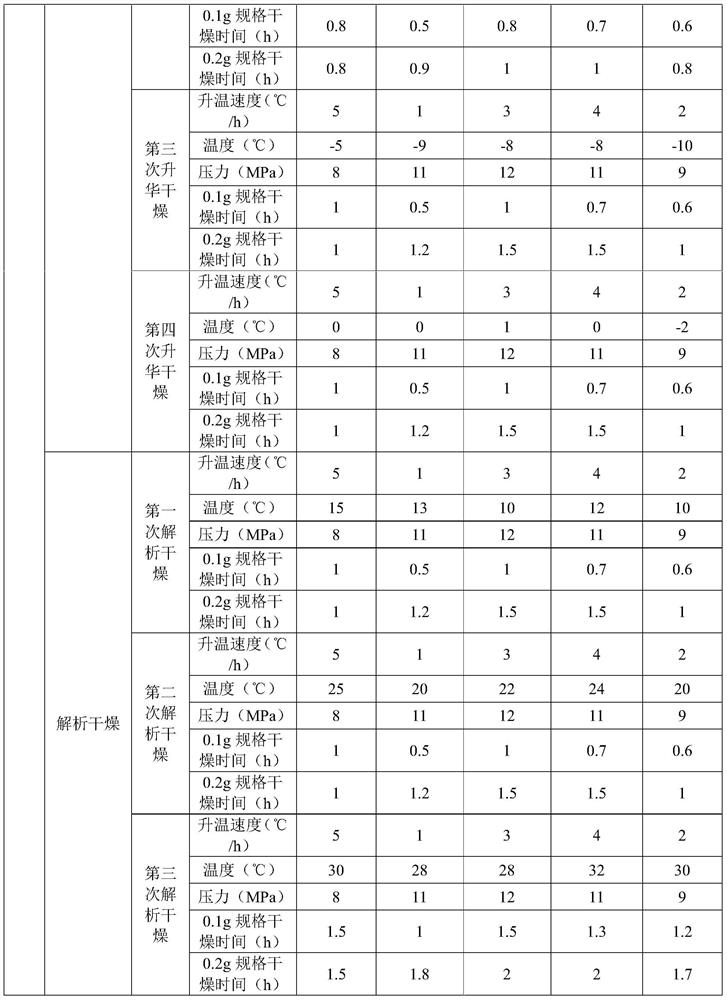
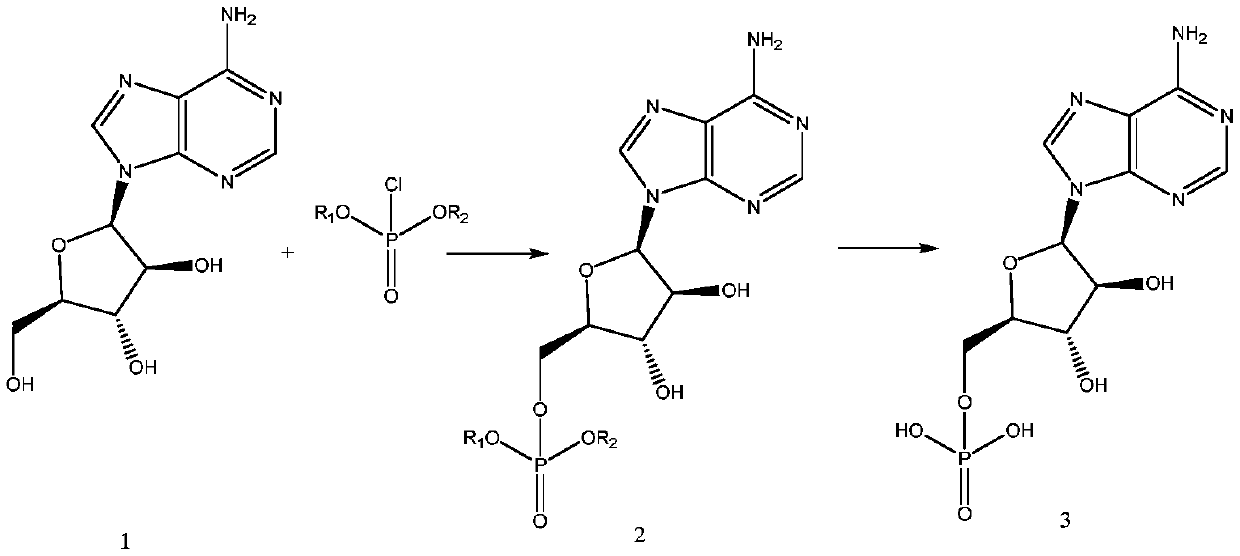
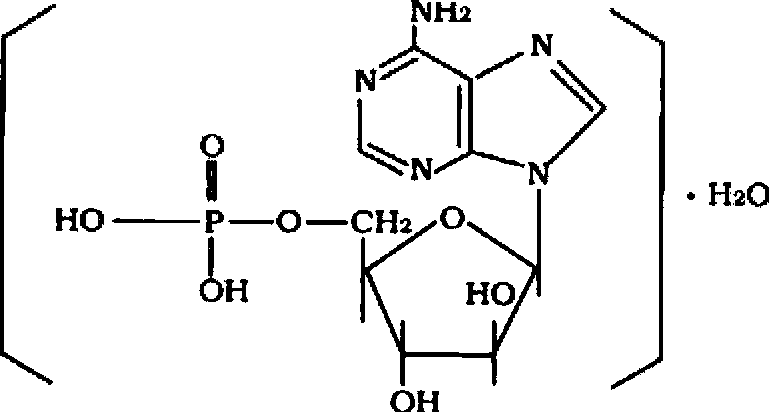
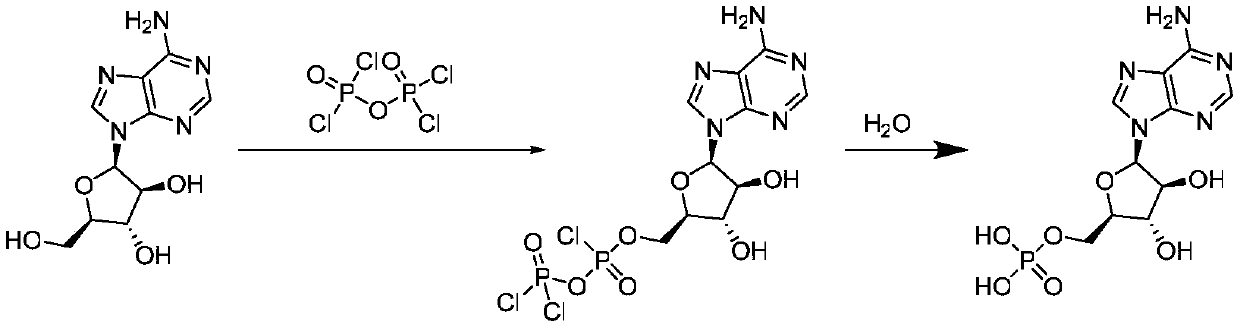
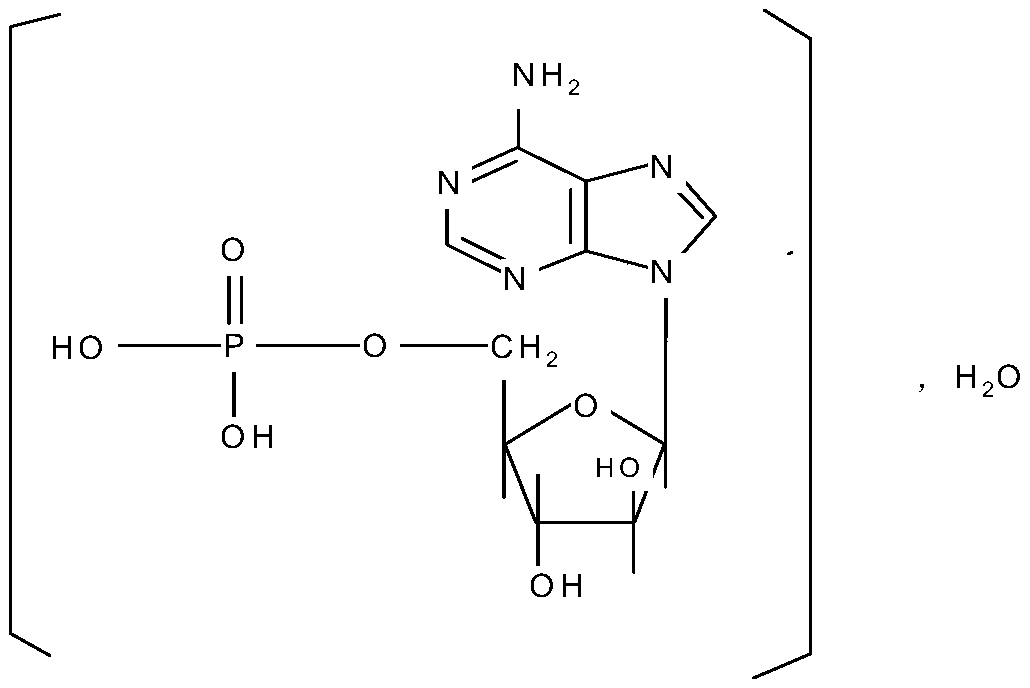
Patents
Literature
34 results about "Vidarabine Monophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
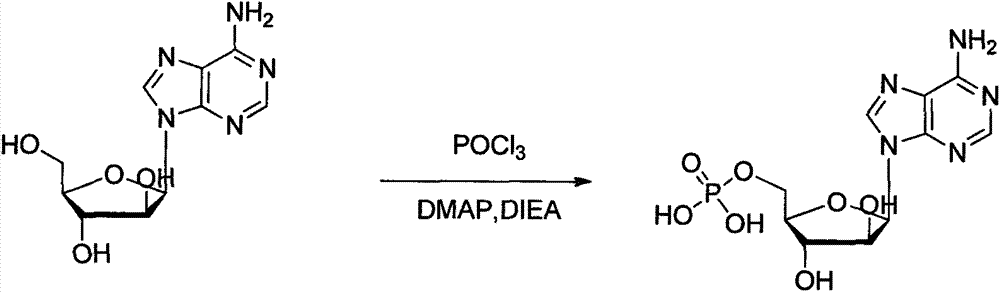
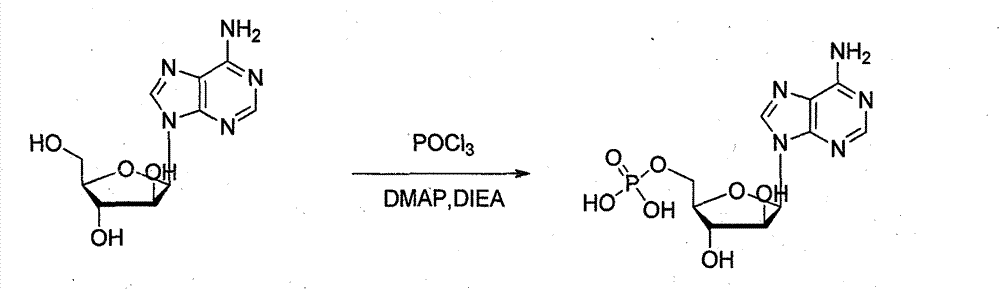
Preparation method of vidarabine monophosphate
InactiveCN102079766ARealize industrial productionRealize the synthesis processSugar derivativesSugar derivatives preparationVidarabine MonophosphateRaw material
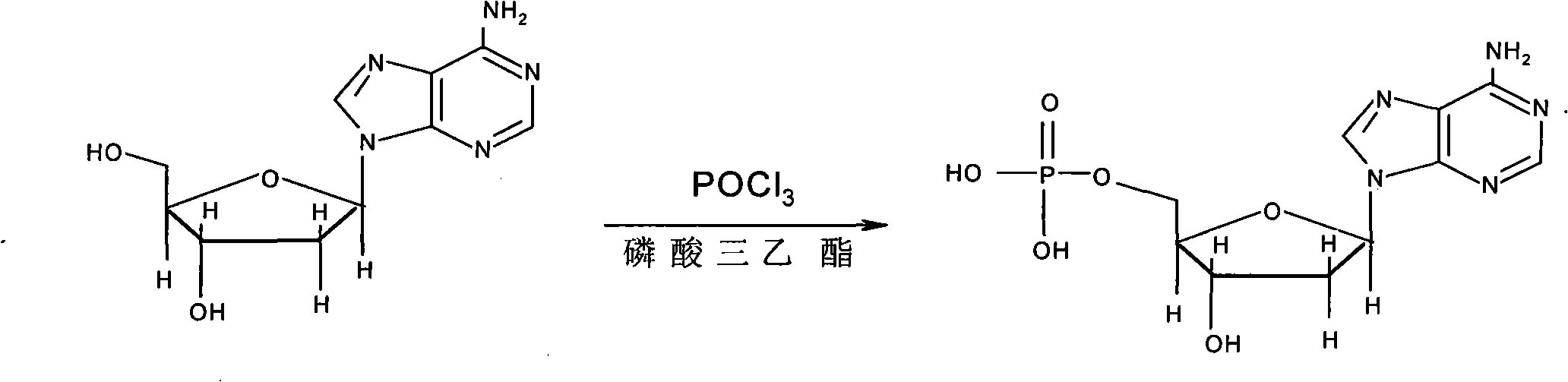
The invention provides a synthesis process of vidarabine monophosphate, which comprises the steps of: dissolving vidarabine as a raw material in triethyl phosphate, adding phosphorus oxychloride for low-temperature reaction through protection, and carrying out posttreatment, such as decoloring with active carbon and the like, to obtain a crude product of the vidarabine monophosphate. The invention has the advantages of short process route, higher yield and reduced reaction cost. The vidarabine monophosphate is prepared with the method which is simple in operation, thus the industrialized production is easy to realize, and the production efficiency is increased.
Owner:双鹤药业(海南)有限责任公司
Vidarabine monophosphate freeze-dried powder injection and preparation method thereof
ActiveCN102379853AGood formabilityHigh clarityPowder deliveryOrganic active ingredientsFreeze-dryingPhosphoric acid
The invention relates to a vidarabine monophosphate freeze-dried powder injection and a preparation method thereof, and the vidarabine monophosphate freeze-dried powder injection is prepared by freeze-drying vidarabine monophosphate, sodium hydroxide solution and water for injection, wherein the sodium hydroxide solution is used as a pH regulator, and the sodium hydroxide solution is used until the pH value of liquid medicine is 7.0-7.5. The vidarabine monophosphate freeze-dried powder injection provided by the invention is few in types of auxiliary materials, low in using amount, good in forming property of a freeze-dried product, good in re-dissolving property, clear in appearance of the solution before freezing, good in clarity after being freeze-dried, low in content of impurities, good in stability and controllable in quality, and can reduce the potential safety hazard of the vidarabine monophosphate freeze-dried powder injection, and improve the efficacy of the vidarabine monophosphate freeze-dried powder injection.
Owner:HAINAN JINRUI PHARMA CO LTD
Method for preparing vidarabine monophosphate for injection
ActiveCN101926777AEasy to producePowder deliveryOrganic active ingredientsFreeze-dryingFurbenicillin
The invention discloses a method for preparing vidarabine monophosphate for injection, which comprises the following steps of: dissolving vidarabine monophosphate into a solvent with heating, adding a proper amount of excipient and active carbon into the solution, filtering the solution to remove carbon, filtering the solution through a micropore filtration membrane, canning the filtrate into a tubular furbenicillin bottle, and putting the furbenicillin bottle into a freeze drier to perform freeze drying. The method is easy for industrialized production, solves the problem of vidarabine monophosphate freeze drying preparation, and ensures stable product quality.
Owner:GUANGDONG XIANQIANG PHARMA +1
Refining method of vidarabine monophosphate
The invention discloses a refining method of vidarabine monophosphate. The refining method is characterized by comprising the following steps of: regulating the pH of a vidarabine monophosphate crude product and an antioxidant to 8-10 by using alkali, and heating and dissolving; then adding a right amount of active carbon, and filtering while hot; regulating pH to 3-5; and crystallizing. The refining method disclosed by the invention can achieve high yield and can be used for refining the high-purity vidarabine monophosphate.
Owner:GUANGDONG XIANQIANG PHARMA
Phosphorylation method for preparing vidarabine monophosphate
ActiveCN103204890AReduce generationReduce pollution damageSugar derivativesSugar derivatives preparationPhosphorylationVidarabine Monophosphate
The invention provides a phosphorylation method for preparing vidarabine monophosphate. The method is specifically as below: using vidarabine as a starting material; conducting phosphorylation on the material under the protection of inert gas and under the effect of catalyst; and conducting active carbon decoloration treatment to obtain a vidarabine monophosphate crude product. The process route is simple, low-cost and high-yield; and compared with the prior art, the production process has significantly reduced toxicity, and is suitable for process production.
Owner:GUANGDONG XIANQIANG PHARMA
Vidarabine monophosphate pharmaceutical composition and preparation method thereof
ActiveCN103054817ASignificant effectLess irritatingOrganic active ingredientsPowder deliveryIrritationPhosphoric acid
The present invention relates to a vidarabine monophosphate pharmaceutical composition and a preparation method thereof. The pharmaceutical composition consists of vidarabine monophosphate, vidarabine, sodium chloride and water-soluble cyclodextrin or a water-soluble cyclodextrin derivative, wherein the mass ratio of vidarabine monophosphate, vidarabine, sodium chloride and water-soluble cyclodextrin or the water-soluble cyclodextrin derivative is 25-35:1-3:1:10-20, preferably 30:2:1:15. The vidarabine monophosphate pharmaceutical composition provided by the present invention has significantly reduced irritation to muscle and blood vessels, and better long-term storage stability. Meanwhile, the vidarabine monophosphate pharmaceutical composition provided by the present invention contains both vidarabine monophosphate and vidarabine, thereby having more significant efficacy. The preparation method for the vidarabine monophosphate pharmaceutical composition is simple and feasible, suitable for industrial production.
Owner:HAINAN JINRUI PHARMA CO LTD
Officinal composition of vidarabine monophosphate for injection
ActiveCN103599080AEasy to controlSpray bottlePowder deliveryOrganic active ingredientsActive componentVidarabine Monophosphate
The invention relates to an officinal composition of vidarabine monophosphate for injection, wherein the main active components of the composition comprise vidarabine monophosphate and lysine.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
Preparation method of vidarabine monophosphate
InactiveCN104372050ABacteriaMicroorganism based processesNucleoside phosphorylaseGenetically engineered
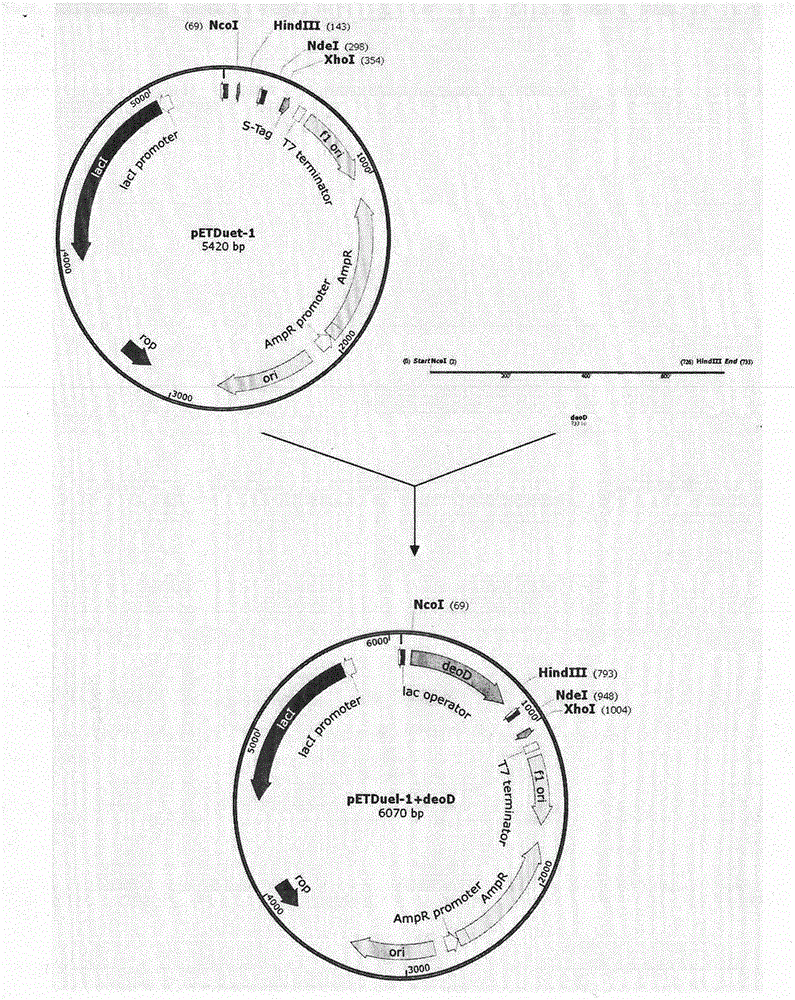
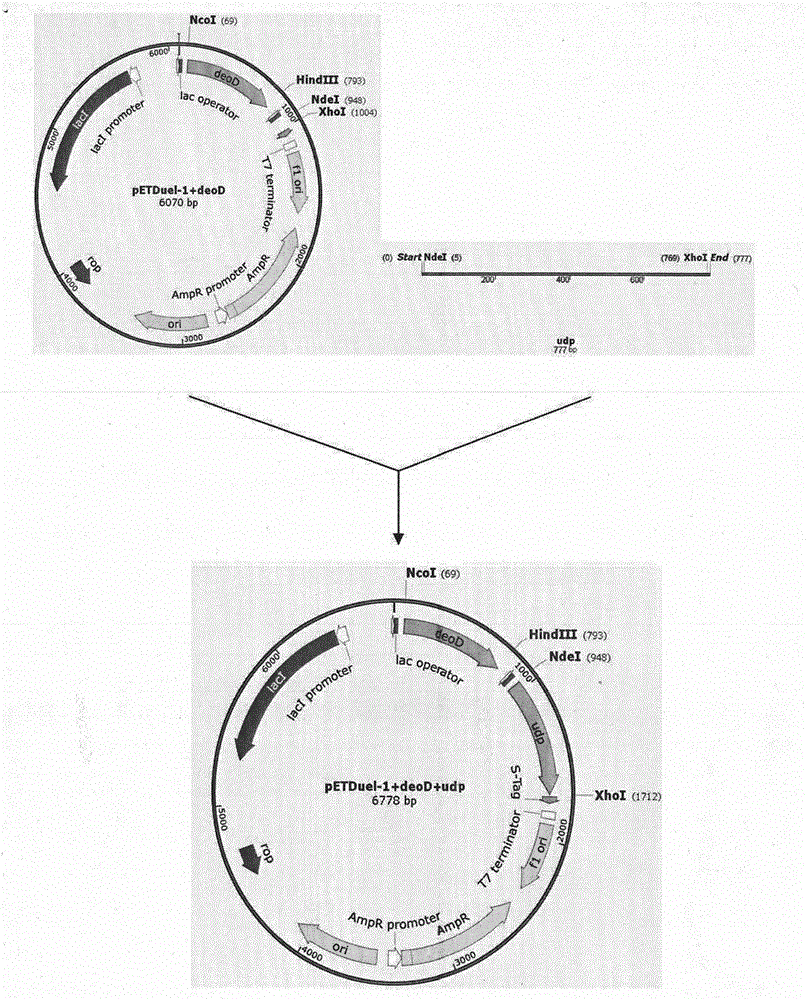
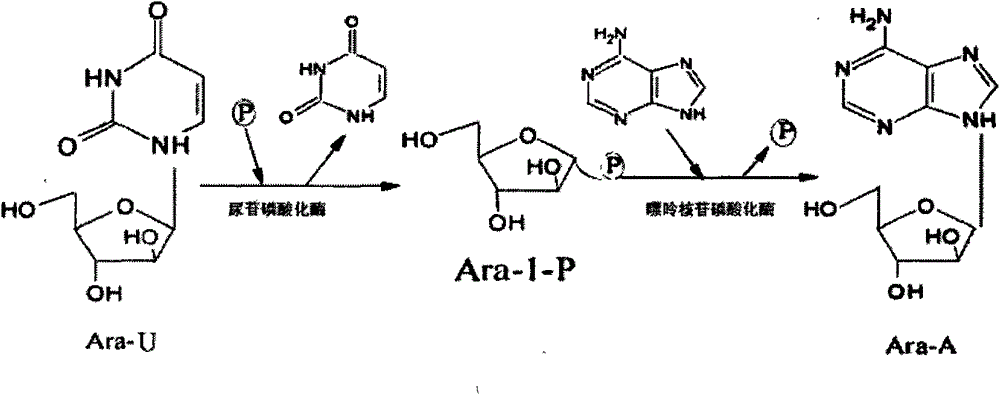
The invention discloses a preparation method of vidarabine monophosphate. The method first prepares genetically engineered bacterium with co-expression of purine nucleoside phosphorylase and uridine phosphorylase, and then uses the genetically engineered bacterium to prepare vidarabine monophosphate. The method is characterized in that coexpression of purine nucleoside phosphorylase and uridine phosphorylase is realized in genetically engineered bacterium by using a molecular cloning method, so as to obtain vidarabine biotransformation bacteria with high enzyme specific activity; the thalli obtained by the invention is used to produce vidarabine; and the reaction substrate concentration can be increased to more than 100 mM, the reaction conversion rate is more than 90%, the dosage of reaction thalli is less than 0.3% (thalli wet weight / reaction volume ml), and reaction time is shortened to 6 h.
Owner:GUANGDONG XIANQIANG PHARMA
Preparation method of vidarabine monophasphate
ActiveCN107793458AReduce generationReduce production processSugar derivativesSugar derivatives preparationTwo stepSide reaction
The invention discloses a preparation method of vidarabine monophosphate. The preparation method comprises the following two steps: crude product synthesis and crude product refining. In a crude product synthesis stage, reaction conditions are controlled severely, side reaction and production of related substances are reduced effectively, and high percent conversion of a target product is ensured.Meanwhile, because most of side products of reaction are dissolved in the reaction system and the target product is not dissolved in the reaction system basically, aftertreatment is facilitated to improve the purity of the product. The method is simple in process, high in product yield, high in purity and high in reaction selectivity; any special equipment is not used during production; and the preparation method is suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Preparation method of vidarabine monophosphate freeze-dried powder injection for injection
InactiveCN102908320AQuality assuranceAvoid influencePowder deliveryOrganic active ingredientsSodium bicarbonateDrugs solution
The invention discloses a preparation method of a vidarabine monophosphate freeze-dried powder injection for injection. The preparation method comprises the steps as follows: taking vidarabine monophosphate, sodium bicarbonate and injection water as raw materials; preparing the raw materials into a vidarabine monophosphate drug solution; canning the drug solution and pressing by half; freezing and drying; pressing; re-pressing; and unpacking to obtain the vidarabine monophosphate freeze-dried powder injection. An excipient or a similar sodium hydroxide strong base solution is not adopted in the adopted vidarabine monophosphate drug solution, so that the influence of the excipient or the similar sodium hydroxide strong base solution on the quality of the vidarabine monophosphate freeze-dried powder injection in the prior art is avoided, the influence of the excipient or the similar sodium hydroxide strong base solution on the vidarabine monophosphate freeze-dried powder injection is effectively reduced, and the quality of drugs is better guaranteed. The vidarabine monophosphate freeze-dried powder injection is better in re-solubility, good in formability and appearance, full and glossy.
Owner:KAIFENG MINGREN PHARMA
Vidarabine monophosphate freeze-dried powder injection for injection and preparation method thereof
InactiveCN107890459AIncrease sublimation speedShorten freeze-drying timePowder deliveryOrganic active ingredientsPorosityDrugs solution
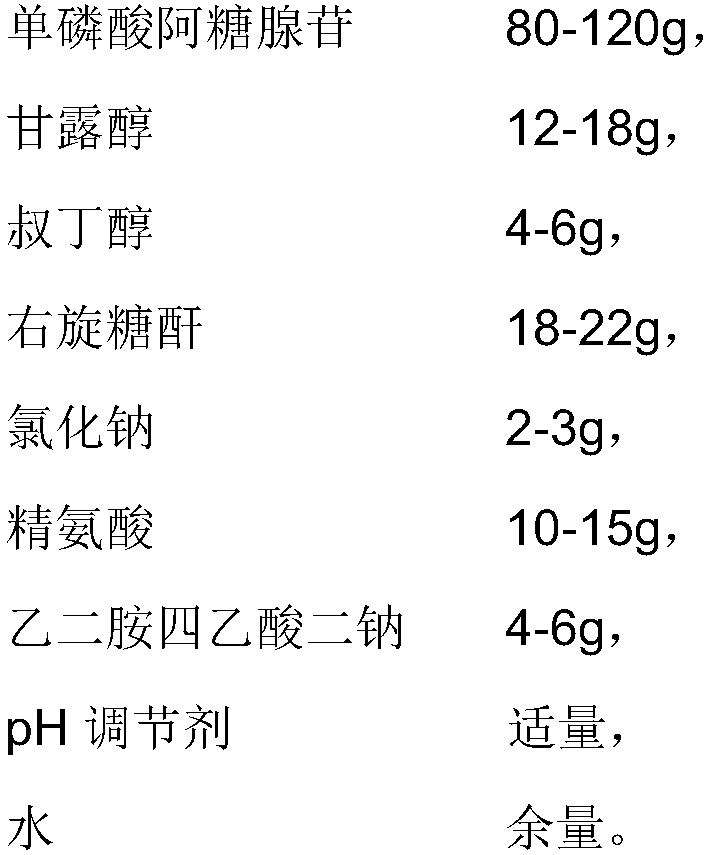
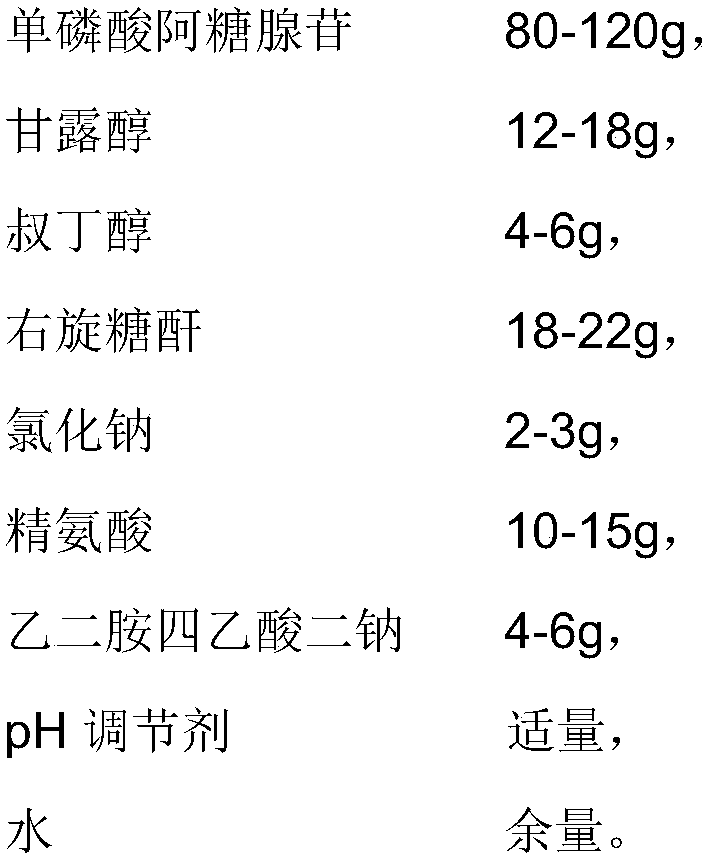
The invention provides a vidarabine monophosphate freeze-dried powder injection for injection; the vidarabine monophosphate freeze-dried powder injection for injection is prepared by freeze-drying a vidarabine monophosphate drug solution, wherein the pH of the vidarabine monophosphate drug solution is 7.0-7.5, and each 2000 mL of the vidarabine monophosphate drug solution contains 80-120 g of adenosine monophosphate, 12-18 g of mannitol, 4-6 g of tert-butanol, 18-22 g of dextran, 2-3 g of sodium chloride, 10-15 g of arginine, 4-6 g of ethylenediamine tetraacetic acid disodium salt, a proper amount of a pH regulator, and the balance water. A proper auxiliary material is added to the formula, so the appearance shape and porosity of the freeze-dried product can be improved, the surface of theproduct is more smooth, moreover, the drug properties are more stable, and the preservation and transportation of the drug are facilitated. Moreover, the preparation process is simple, the technicalrequirements are not high, and the production cycle is relatively short; the quality of the prepared product is stable and controllable, so as to be good for long-term storage.
Owner:HAINAN GENERAL & KANGLI PHARMA
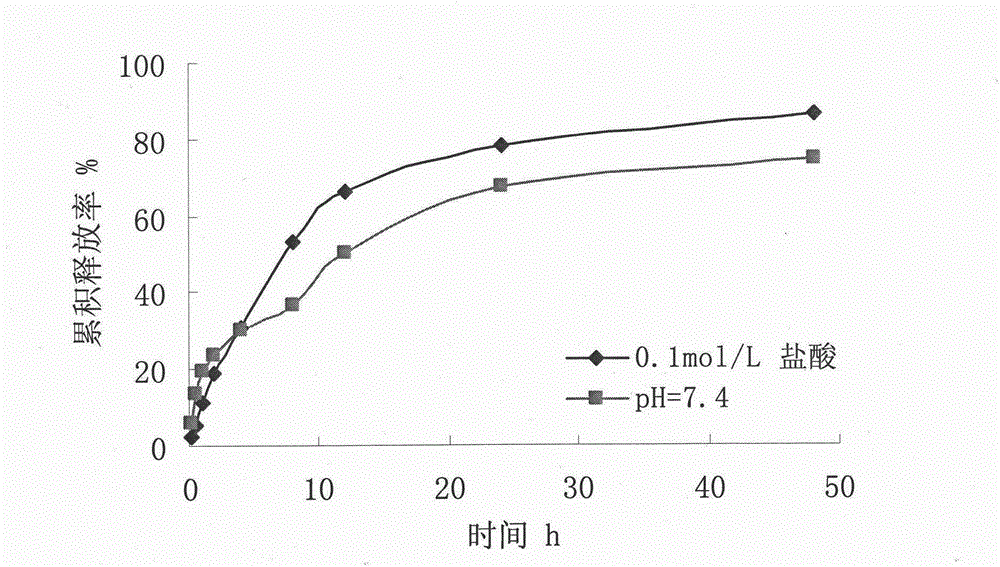
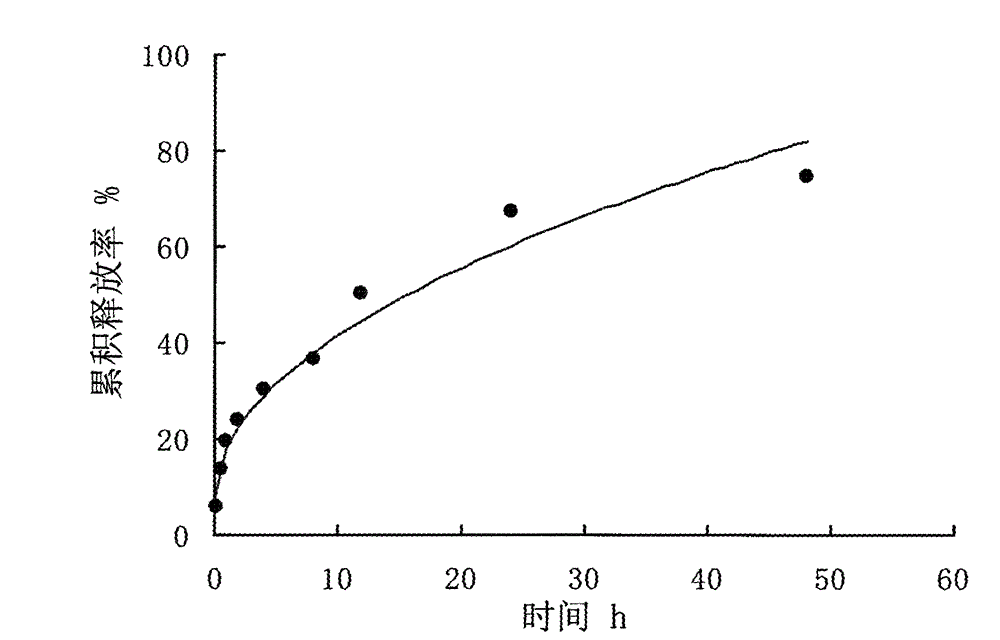
Vidarabine monophosphate microsphere drug delivery system and preparation method thereof
ActiveCN103142491ANo obvious adhesionGood dispersionOrganic active ingredientsAntiviralsDispersityPolyethylene glycol
The invention relates to a chitosan microsphere drug delivery system of vidarabine and a preparation method. The method is characterized by acquiring the the medicinal microsphere drug delivery system with a complete spherical shape, a uniform particle size, a high embedding rate, good dispersity and a sustained release property. The specific technical scheme is that: chitosan is taken as a membrane material, polyethylene glycol is adopted as a pore-forming agent, glutaraldehyde is employed as a crosslinking agent, and inverse emulsification-crosslinking method is used to prepare the drug carrying microspheres. The preparation method includes: dissolving the drug in an acidic aqueous solution of chitosan, adding polyethylene glycol and stirring them uniformly, and taking the mixture as an aqueous phase; adding the aqueous phase slowly into an oil phase containing a surface active agent and a dispersing agent, carrying out stirring and emulsifying to uniformity, and adding the crosslinking agent to undergo crosslinking solidification; and washing and drying the obtained product. The invention provides the vidarabine chitosan microsphere drug delivery system with a sustained-release property.
Owner:GUANGDONG XIANQIANG PHARMA +1
Synthetic method of vidarabine monophosphate
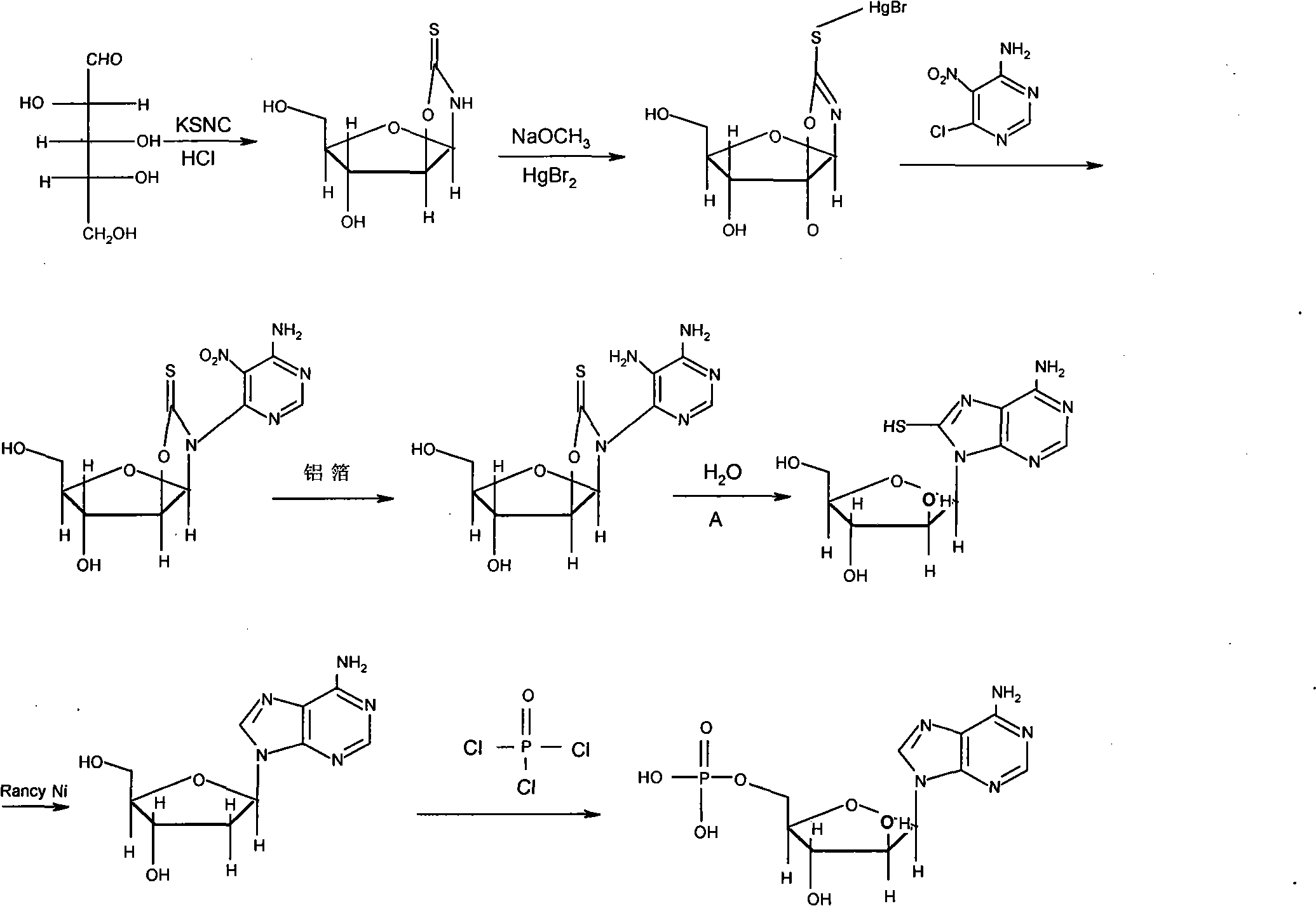
ActiveCN107056862AReduce usageRelieve pressureSugar derivativesSugar derivatives preparationSodium bisulfideSodium hydrosulfide
The invention provides a synthetic method of vidarabine monophosphate. The synthetic method comprises the following steps: a) making an intermediate shown as a formula (II) react with a solid sulfurizing agent and strong-acid ion exchange resin to obtain an intermediate shown as a formula (III), wherein the solid sulfurizing agent is selected from sodium sulfide, a sodium sulfide hydrate, sodium hydrosulfide or a sodium hydrosulfide hydrate; b), desulfurizing the intermediate shown as the formula (III) to obtain the vidarabine monophosphate. In the method, the intermediate shown as the formula (II) is subjected to sulfhydrylation through the solid sulfurizing agent such as the sodium sulfide, the sodium bisulfide, the sodium sulfide hydrate or the sodium hydrosulfide hydrate and the strong-acid ion exchange resin, and is desulfurized to obtain the vidarabine monophosphate, so that the use of hydrogen sulfide is avoided, and the pressure on the environment is lowered. Meanwhile, by adopting the synthetic method provided by the invention, the yield is 30 percent or more, and the purity of an obtained product is 99.8 percent or more.
Owner:GANSU CHANGEE BIO PHARMA
Vidarabine monophosphate for injection and method for producing vidarabine monophosphate
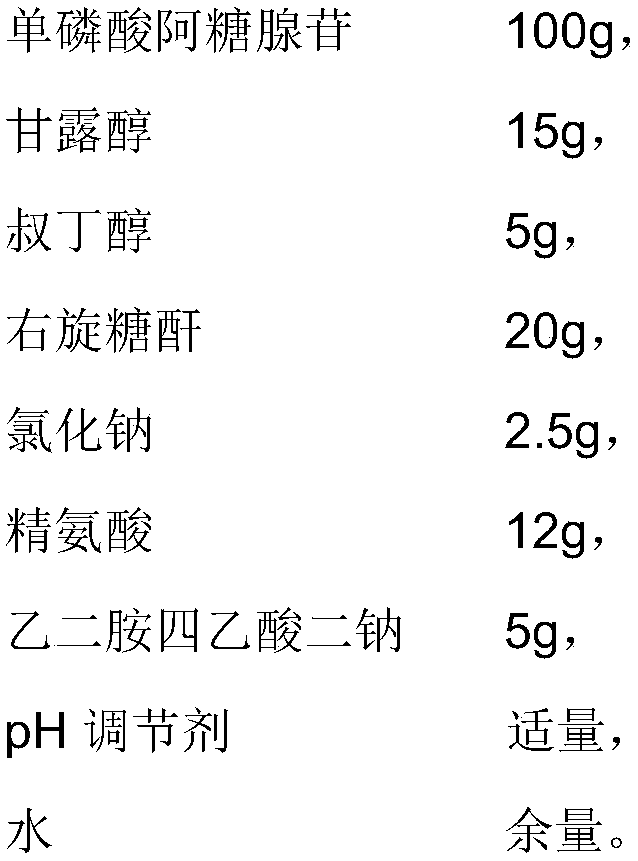
InactiveCN103536445AGood crystal formSimple stepsPharmaceutical product form changeMANNITOL/SORBITOLFreeze-drying
The invention discloses vidarabine monophosphate for injection and a method for producing the vidarabine monophosphate. The vidarabine monophosphate for the injection comprises 100.0g of the vidarabine monophosphate, 50.0g of mannitol, 5.0g of medicinal carbon, and 2000ml of water for the injection. The method sequentially comprises the following steps: (1) washing, drying and disinfecting a bottle, (2) washing and disinfecting a plug, (3) conducting preparation, (4) conducting filling, (5) conducting freeze drying, (6) rolling a cover, (7) conducting light inspection, and (8) conducting lettering, packaging and storage. Compared with the prior art, the vidarabine monophosphate produced by the method is good in crystal form, simple in step and short in cycle, and the production efficiency is improved.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
A kind of refining method of vidarabine monophosphate
The invention discloses a refining method of vidarabine monophosphate. The refining method is characterized by comprising the following steps of: regulating the pH of a vidarabine monophosphate crude product and an antioxidant to 8-10 by using alkali, and heating and dissolving; then adding a right amount of active carbon, and filtering while hot; regulating pH to 3-5; and crystallizing. The refining method disclosed by the invention can achieve high yield and can be used for refining the high-purity vidarabine monophosphate.
Owner:GUANGDONG XIANQIANG PHARMA
Production process of vidarabine monophosphate
ActiveCN109134569AReduce in quantityHigh yieldSugar derivativesSugar derivatives preparationOrganic solventPalladium catalyst
The invention belongs to the technical field of preparation of vidarabine monophosphate and particularly relates to a production process of vidarabine monophosphate. The production process of vidarabine monophosphate comprises the following steps of S1 dissolving vidarabine into organic solvent and cooling down the mixture; S2, adding in phosphoryl chloride to perform thermal reaction until that the residual content of the vidarabine is not higher than 3% of the added amount; S3, adding in palladium catalysts for catalytic reduction, then filtering reaction products, removing the solvent fromthe obtained filter liquor to obtain crude vidarabine monophosphate; S4, recrystallizing the crude vidarabine monophosphate obtained in S3 for purification. The production process of vidarabine monophosphate introduces the phosphoryl chloride relatively low in reactivity to react with the raw vidarabine, the reaction process is mild and easy to control, so that the produced quantity of side products can be reduced from the source; meanwhile, the novel palladium catalysts can selectively reduce introduced ether groups, thereby simplifying purification processes and achieving high product yieldand purity.
Owner:海南卓科制药有限公司
Vidarabine monophosphate injection and preparation method thereof
InactiveCN103202804AEasy to useEnsure safetyOrganic active ingredientsPharmaceutical delivery mechanismUse medicationVidarabine Monophosphate
The invention provides a vidarabine monophosphate injection and a preparation method thereof. The injection is prepared from the following raw materials by weight: 50-200 parts of vidarabine monophosphate, 0.015-0.05 part of a pH regulator and 2000 parts of water for injection. A finished product prepared by the method provided by the invention has low impurity content, good homogeneity and good stability. A water injection is subjected to terminal sterilization to ensure the safety of the product. The water injection is convenient to use, and does not need to be prepared before clinical application, so as to reduce the potential safety hazard caused by operation and provide a new choice of medication for clinical use.
Owner:GUANGDONG XIANQIANG PHARMA
Vidarabine monophosphate pharmaceutical composition
InactiveCN105232571AFreeze-drying time is not longShorten the production cyclePowder deliveryOrganic active ingredientsSide effectHerpesvirus infection
The invention relates to a vidarabine monophosphate pharmaceutical composition and a preparation method thereof. A low dose of the vidarabine monophosphate pharmaceutical composition is effective in treating herpesvirus infection or cytomegalovirus infection in children 0 to 14 years old, and the toxic and side effect and the increase in medical cost caused by a high-dose or ultrahigh-dose administering manner of 5-10 mg / kg reported in documents are avoided.
Owner:双鹤药业(海南)有限责任公司
Synthesis technology of vidarabine monophosphate
InactiveCN106866763AHigh purityHigh yieldSugar derivativesSugar derivatives preparationChlorideSolvent
The invention discloses a synthesis technology of vidarabine monophosphate. The synthesis technology comprises the following steps: suspension dissolving vidarabine into an aprotic polar solvent firstly, carrying out reaction on a reactant and pyrophosphoryl chloride at low temperature, then carrying out hydrolysis quenching, regulating a pH value to 2.5-3, and precipitating the vidarabine monophosphate, wherein the purity of a crude product is 95 percent or above; and finally, carrying out recrystallization treatment on the vidarabine monophosphate to improve the purity of the vidarabine monophosphate to 99 percent or above with the maximum single impurity content controlled to be 0.2 percent or below. The synthesis technology of the invention has the advantages of high yield and less impurities and is suitable for industrial production.
Owner:SHANGHAI INST OF TECH
Vidarabine monophosphate freeze-dried powder injection and preparation method thereof
ActiveCN102379853BGood formabilityHigh clarityOrganic active ingredientsPowder deliveryFreeze-dryingPhosphoric acid
The invention relates to a vidarabine monophosphate freeze-dried powder injection and a preparation method thereof, and the vidarabine monophosphate freeze-dried powder injection is prepared by freeze-drying vidarabine monophosphate, sodium hydroxide solution and water for injection, wherein the sodium hydroxide solution is used as a pH regulator, and the sodium hydroxide solution is used until the pH value of liquid medicine is 7.0-7.5. The vidarabine monophosphate freeze-dried powder injection provided by the invention is few in types of auxiliary materials, low in using amount, good in forming property of a freeze-dried product, good in re-dissolving property, clear in appearance of the solution before freezing, good in clarity after being freeze-dried, low in content of impurities, good in stability and controllable in quality, and can reduce the potential safety hazard of the vidarabine monophosphate freeze-dried powder injection, and improve the efficacy of the vidarabine monophosphate freeze-dried powder injection.
Owner:HAINAN JINRUI PHARMA CO LTD
A method for preparing vidarabine monophosphate by phosphorylation
ActiveCN103204890BReduce solubilityHigh yieldSugar derivativesSugar derivatives preparationPhosphorylationVidarabine Monophosphate
The invention provides a phosphorylation method for preparing vidarabine monophosphate. The method is specifically as below: using vidarabine as a starting material; conducting phosphorylation on the material under the protection of inert gas and under the effect of catalyst; and conducting active carbon decoloration treatment to obtain a vidarabine monophosphate crude product. The process route is simple, low-cost and high-yield; and compared with the prior art, the production process has significantly reduced toxicity, and is suitable for process production.
Owner:GUANGDONG XIANQIANG PHARMA
Vidarabine monophosphate microsphere drug delivery system and preparation method thereof
The invention relates to a chitosan microsphere drug delivery system of vidarabine and a preparation method. The method is characterized by acquiring the the medicinal microsphere drug delivery system with a complete spherical shape, a uniform particle size, a high embedding rate, good dispersity and a sustained release property. The specific technical scheme is that: chitosan is taken as a membrane material, polyethylene glycol is adopted as a pore-forming agent, glutaraldehyde is employed as a crosslinking agent, and inverse emulsification-crosslinking method is used to prepare the drug carrying microspheres. The preparation method includes: dissolving the drug in an acidic aqueous solution of chitosan, adding polyethylene glycol and stirring them uniformly, and taking the mixture as an aqueous phase; adding the aqueous phase slowly into an oil phase containing a surface active agent and a dispersing agent, carrying out stirring and emulsifying to uniformity, and adding the crosslinking agent to undergo crosslinking solidification; and washing and drying the obtained product. The invention provides the vidarabine chitosan microsphere drug delivery system with a sustained-release property.
Owner:GUANGDONG XIANQIANG PHARMA +1
Preparation method of vidarabine monophosphate freeze-dried powder injection for injection
ActiveCN113171347AImprove solubilityImprove stabilityPowder deliveryOrganic active ingredientsPhysical chemistryVidarabine Monophosphate
The invention provides a preparation method of vidarabine monophosphate freeze-dried powder injection for injection, which belongs to the technical field of medicine preparation, and comprises the following steps: taking water for injection, adjusting the pH value to 8.0-8.5, adding vidarabine monophosphate for dissolving, decoloring, degerming, filtering, sub-packaging, pre-freezing the obtained feed liquid, sublimating and drying for four times, desorbing and drying for three times, and obtaining the vidarabine monophosphate freeze-dried powder injection for injection. By adjusting the pH value, the hydrolysis reaction of vidarabine monophosphate is reduced, and the impurity content of the finished product is reduced; through setting of technological parameters in the sublimation drying process and the desorption drying process, solute migration in liquid medicine is prevented, fullness and looseness of the finished product are guaranteed, and the surface of the finished product is smoother. The preparation method provided by the invention can effectively improve the drug stability, appearance shape and looseness of the finished product.
Owner:HAINAN JINRUI PHARMA
A kind of production technology of vidarabine monophosphate
ActiveCN109134569BReduce in quantityHigh yieldSugar derivativesSugar derivatives preparationPhosphoric acidPalladium catalyst
The invention belongs to the technical field of preparation of vidarabine monophosphate and particularly relates to a production process of vidarabine monophosphate. The production process of vidarabine monophosphate comprises the following steps of S1 dissolving vidarabine into organic solvent and cooling down the mixture; S2, adding in phosphoryl chloride to perform thermal reaction until that the residual content of the vidarabine is not higher than 3% of the added amount; S3, adding in palladium catalysts for catalytic reduction, then filtering reaction products, removing the solvent fromthe obtained filter liquor to obtain crude vidarabine monophosphate; S4, recrystallizing the crude vidarabine monophosphate obtained in S3 for purification. The production process of vidarabine monophosphate introduces the phosphoryl chloride relatively low in reactivity to react with the raw vidarabine, the reaction process is mild and easy to control, so that the produced quantity of side products can be reduced from the source; meanwhile, the novel palladium catalysts can selectively reduce introduced ether groups, thereby simplifying purification processes and achieving high product yieldand purity.
Owner:海南卓科制药有限公司
Vidarabine monophosphate freeze-dried powder injection for injection and preparation method thereof
ActiveCN101642440BProduct quality is stable and controllableSimple operation processOrganic active ingredientsPowder deliveryDrugs solutionMANNITOL/SORBITOL
The invention relates to a vidarabine monophosphate freeze-dried powder injection for injection and a preparation method thereof. The freeze-dried powder injection is obtained by freeze-drying a vidarabine monophosphate drug solution. The formula is: 100 g of vidarabine monophosphate, 25 g of mannitol, 3 g of disodium hydrogen phosphate, 0.3 g of sodium dihydrogen phosphate, 1 g of disodium edetate, and add water for injection to 2000 mL. The freeze-drying process in the preparation method of the freeze-dried powder injection includes three processes of pre-freezing, sublimation and temperature-rising drying. The freeze-dried powder injection and the preparation method thereof of the present invention have significant advantages in storage and transportation compared with the commonly used water injection in the prior art.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
Synthesis method and application of vidarabine monophosphate
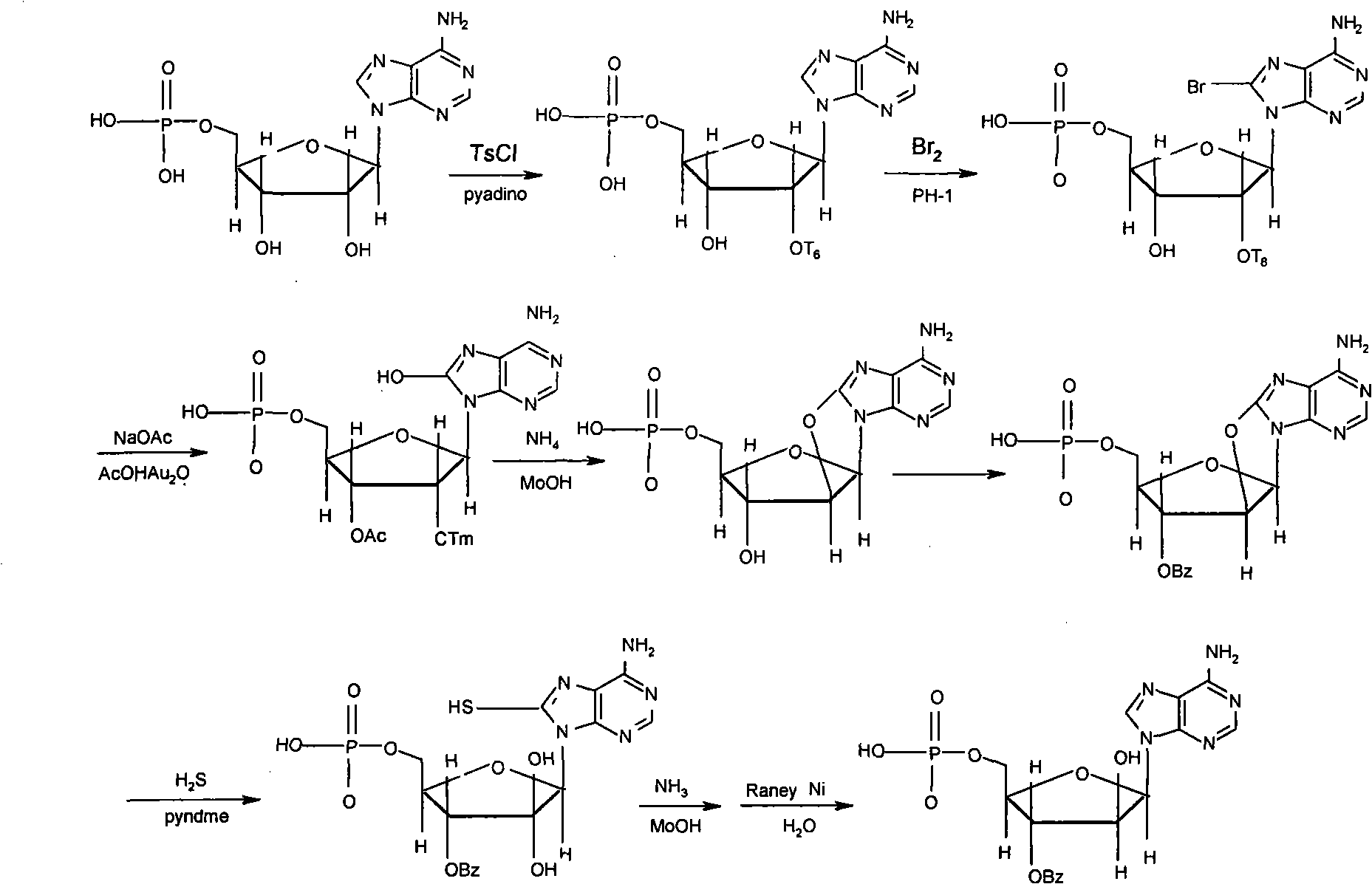
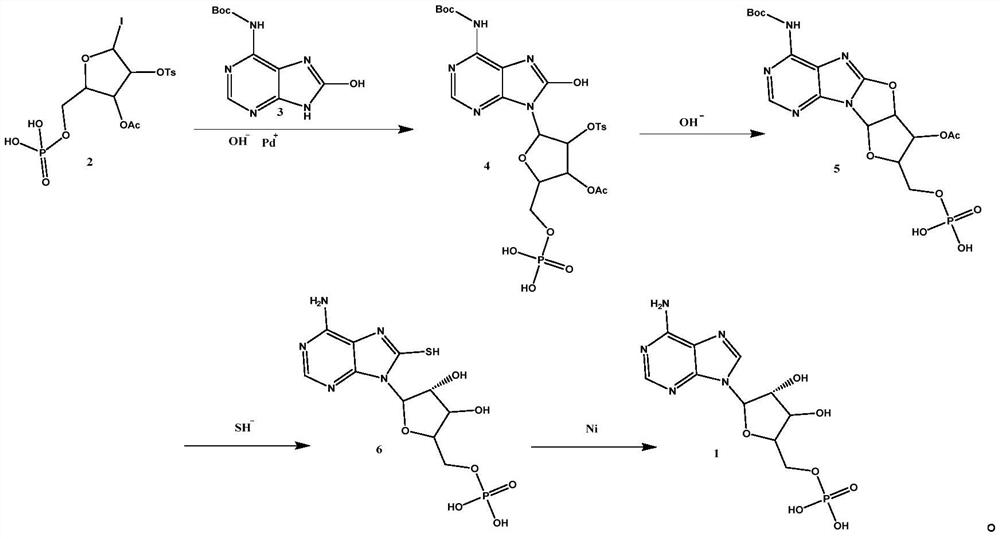
PendingCN113173957AReduce the introductionInhibit synthesisSugar derivativesPharmaceutical delivery mechanismCarbamatePurine
The invention belongs to the field of medicine synthesis, and discloses a synthesis method and application of vidarabine monophosphate. According to the synthesis method, 5-iodo-2-((phosphonooxy) methyl)-4-(tosyloxy)tetrahydrofuran-3-yl acetate and tert-butyl (8-hydroxy-9H-purin-6-yl)carbamate are subjected to condensation, epoxidation, ring opening and desulfurization reaction, and the vidarabine monophosphate is synthesized. According to the synthesis method of vidarabine monophosphate, provided by the invention, the industrial production steps are further simplified, the total reaction yield is improved, and the industrial production cost is reduced. The method is suitable for synthesizing vidarabine monophosphate, and the synthesized vidarabine monophosphate is used for preparing vidarabine monophosphate for injection.
Owner:HAINAN JINRUI PHARMA
A kind of medicinal composition of vidarabine monophosphate for injection
ActiveCN103599080BFlat surfaceNot crackedPowder deliveryOrganic active ingredientsMedicineActive component
The invention relates to an officinal composition of vidarabine monophosphate for injection, wherein the main active components of the composition comprise vidarabine monophosphate and lysine.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
Vidarabine monophosphate pharmaceutical composition and preparation method thereof
ActiveCN103054817BSignificant effectLess irritatingPowder deliveryOrganic active ingredientsIrritationPhosphoric acid
The present invention relates to a vidarabine monophosphate pharmaceutical composition and a preparation method thereof. The pharmaceutical composition consists of vidarabine monophosphate, vidarabine, sodium chloride and water-soluble cyclodextrin or a water-soluble cyclodextrin derivative, wherein the mass ratio of vidarabine monophosphate, vidarabine, sodium chloride and water-soluble cyclodextrin or the water-soluble cyclodextrin derivative is 25-35:1-3:1:10-20, preferably 30:2:1:15. The vidarabine monophosphate pharmaceutical composition provided by the present invention has significantly reduced irritation to muscle and blood vessels, and better long-term storage stability. Meanwhile, the vidarabine monophosphate pharmaceutical composition provided by the present invention contains both vidarabine monophosphate and vidarabine, thereby having more significant efficacy. The preparation method for the vidarabine monophosphate pharmaceutical composition is simple and feasible, suitable for industrial production.
Owner:HAINAN JINRUI PHARMA CO LTD
A kind of synthetic technique of vidarabine monophosphate
InactiveCN106866763BHigh purityHigh yieldSugar derivativesSugar derivatives preparationChlorideAdenosine 5 monophosphate
The invention discloses a synthesis technology of vidarabine monophosphate. The synthesis technology comprises the following steps: suspension dissolving vidarabine into an aprotic polar solvent firstly, carrying out reaction on a reactant and pyrophosphoryl chloride at low temperature, then carrying out hydrolysis quenching, regulating a pH value to 2.5-3, and precipitating the vidarabine monophosphate, wherein the purity of a crude product is 95 percent or above; and finally, carrying out recrystallization treatment on the vidarabine monophosphate to improve the purity of the vidarabine monophosphate to 99 percent or above with the maximum single impurity content controlled to be 0.2 percent or below. The synthesis technology of the invention has the advantages of high yield and less impurities and is suitable for industrial production.
Owner:SHANGHAI INST OF TECH
A kind of preparation method of adenosine monophosphate arabinoside
ActiveCN107793458BReduce generationReduce production processSugar derivativesSugar derivatives preparationBiotechnologyBiochemical engineering
The invention discloses a preparation method of vidarabine monophosphate. The preparation method comprises the following two steps: crude product synthesis and crude product refining. In a crude product synthesis stage, reaction conditions are controlled severely, side reaction and production of related substances are reduced effectively, and high percent conversion of a target product is ensured.Meanwhile, because most of side products of reaction are dissolved in the reaction system and the target product is not dissolved in the reaction system basically, aftertreatment is facilitated to improve the purity of the product. The method is simple in process, high in product yield, high in purity and high in reaction selectivity; any special equipment is not used during production; and the preparation method is suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com