Vidarabine monophosphate freeze-dried powder injection for injection and preparation method thereof
A technology for freeze-dried powder of adenosine monophosphate and adenosine monophosphate, which is applied in the directions of freeze-dried delivery, medical preparations containing active ingredients, powder delivery, etc., which can solve the problem of high technical content requirements and complicated operation processes , long production cycle and other problems, to achieve the effect of high technical requirements, simple preparation process and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
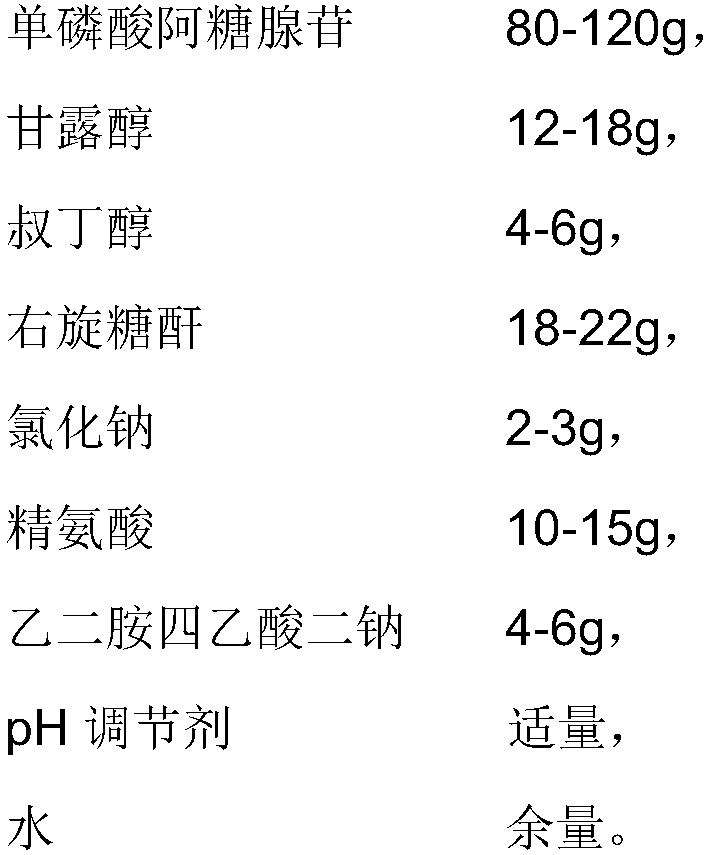
[0029] 1. Prescription
[0030]
[0031] 2. Production process
[0032] 2.1 Control the cleaning and sterilization of antibiotic glass bottles
[0033] Controlled antibiotic glass bottles are removed from the outer packaging, passed into the bottle washing and drying room, cleaned by a bottle washing machine, dried with compressed air, dried and sterilized in a hot air tunnel oven at 350°C for 5 minutes, and cooled.
[0034] 2.2 Cleaning and sterilization of butyl rubber
[0035] The butyl rubber stoppers are removed from the outer packaging and sent to the stopper washing room, cleaned by a stopper washing machine, sterilized by dry heat at 121°C for 120 minutes, and cooled for later use.
[0036] 2.3 Sterilization of aluminum-plastic combination caps
[0037] The aluminum-plastic cover is removed from the outer packaging, and then transferred to the aluminum cover sterilization room, sterilized by dry heat at 110°C for 120 minutes in a drying oven, cooled, and set asid...
Embodiment 2
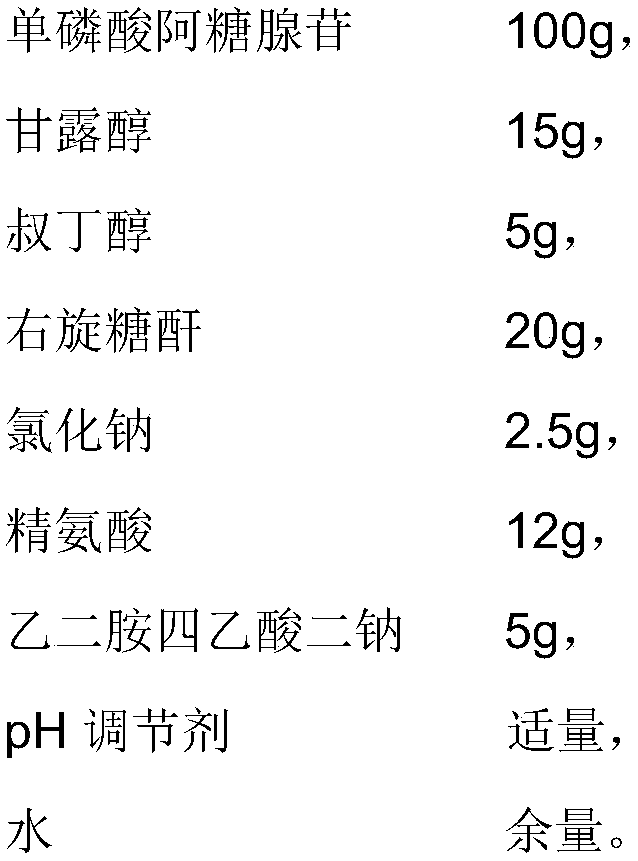
[0049] The difference between the present embodiment and embodiment 1 is that the prescription is different, and the prescription of the present embodiment is as follows:
[0050]
[0051]
Embodiment 3
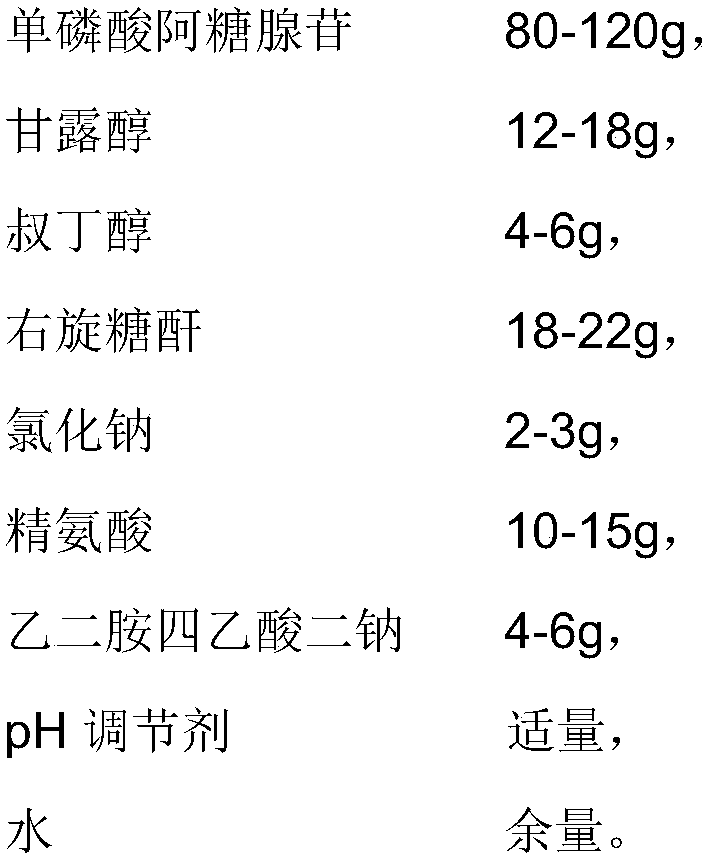
[0053] The difference between the present embodiment and embodiment 1 is that the prescription is different, and the prescription of the present embodiment is as follows:
[0054]
[0055] Stability study
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com