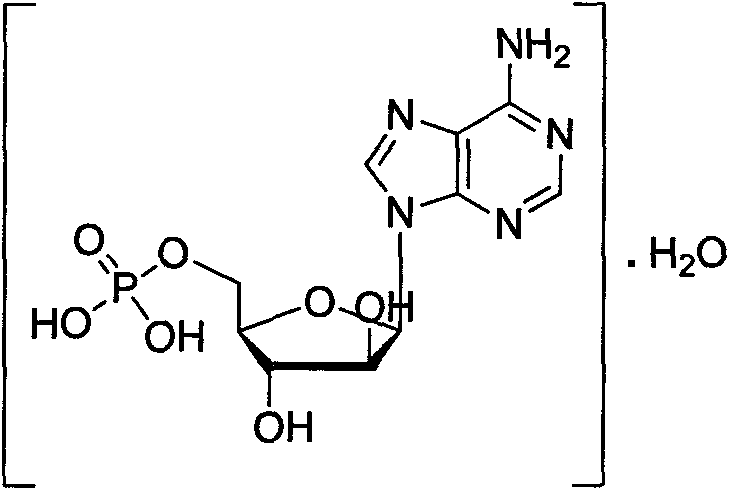
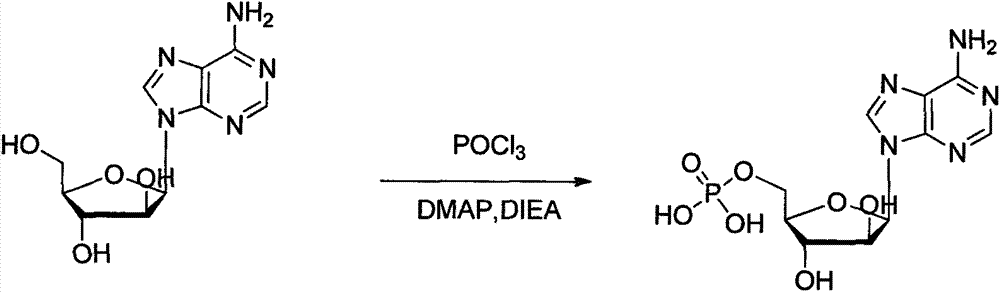
Phosphorylation method for preparing vidarabine monophosphate
A technology of adenosine vidarabine monophosphate and adenosine vidarabine, which is applied in the field of medicine and chemical industry, can solve the problems of high cost, heavy environmental pollution, and difficulty in recycling, and achieve the goal of reducing the generation of impurities, reducing pollution and damage, and improving product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] ①Put 100g (0.35mol) of vidarabine into the reaction flask, add 300ml of dichloromethane, protect with nitrogen gas, add 150ml of N,N-diisopropylethylamine (DIEA) dropwise, add 4-dimethylamino Pyridine (DMAP) 5g, cooled to -10°C to 5°C, and stirred.
[0028] ②Quick stirring step ①Reaction solution, slowly dropwise add 214.9g (1.4mol) of phosphorus oxychloride and 50ml of dichloromethane respectively, after the dropwise addition is completed, keep it at -10°C-5°C for 2h, after the reaction is completed, filter to obtain a solid 173.1g.
[0029] ③Add water to the solid and stir to heat up to 60°C to dissolve, add activated carbon to decolorize, filter, cool the filtrate, keep it at -5°C-0°C for crystallization overnight, filter, rinse the wet product with purified water to obtain 113.9g of crude product, yield 89.1%.
[0030] Add 1140ml of purified water to the reaction bottle of the crude product, heat to 80°C, stir for 15 minutes, cool down to 5°C-0°C and keep warm for...
Embodiment 2
[0032] ①Put 100g (0.35mol) of vidarabine into the reaction flask, add 300ml of dichloromethane, protect with nitrogen gas, add 200ml of N,N-diisopropylethylamine (DIEA) dropwise, add 4-dimethylamino Pyridine (DMAP) 5g, cooled to -10°C to 5°C, and stirred.
[0033] ②Quick stirring step ①Reaction solution, slowly dropwise add 161.2g (1.05mol) of phosphorus oxychloride and 50ml of dichloromethane respectively, after the dropwise addition, keep it at -5°C-0°C for 4h, after the reaction is completed, filter to obtain a solid 156.4 g.
[0034] ③Add water to the solid and stir to heat up to 90°C to dissolve, add activated carbon to decolorize, filter, cool the filtrate, keep it at -5°C-0°C for crystallization overnight, filter, rinse the wet product with purified water to obtain 117.6g of crude product, yield 92%.
[0035] Add 1170ml of purified water to the crude product in a reaction bottle, heat to 80°C, stir for 15 minutes, cool down to 5°C-0 and keep warm for 12 hours, precipi...
Embodiment 3
[0037] ① Put 80g (0.28mol) of vidarabine into the reaction flask, add 200ml of dichloromethane, pass nitrogen gas for protection, add 150ml of N,N-diisopropylethylamine (DIEA) dropwise, add 4-dimethylamino Pyridine (DMAP) 4g, cooled to -10°C to 5°C, and stirred.
[0038] ②Quick stirring step ①Reaction solution, slowly dropwise add 214.9g (1.4mol) of phosphorus oxychloride and 40ml of dichloromethane respectively, after the dropwise addition is completed, keep it at -10°C-5°C for 2h, after the reaction is completed, filter to obtain a solid 173.1 g.
[0039] ③Add water to the solid and stir to heat up to 60°C to dissolve, add activated carbon to decolorize, filter, cool the filtrate, keep it at -5°C-0°C for crystallization overnight, filter, rinse the wet product with purified water to obtain 93.63g of crude product, yield 91.4%.
[0040] Add 936ml of purified water to the crude product, heat to 80°C, stir for 15 minutes, cool down to 5°C-0°C and keep warm for 12 hours, preci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com