Vidarabine monophosphate injection and preparation method thereof
A vidarabine monophosphate injection and the technology of vidarabine monophosphate are applied in the field of pharmaceutical inventions, which can solve the problems of unguaranteed stability and difficult quality control of preparations, and reduce potential safety hazards, ensure safety, and use convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
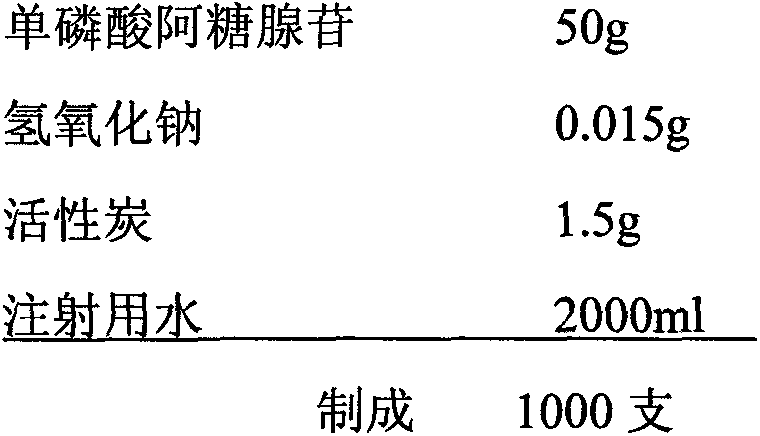
[0023]
[0024] Weigh 50g of vidarabine monophosphate and 0.015g of sodium hydroxide respectively, and set aside; weigh 1.5g of activated carbon, add water for injection about 3 times the amount of activated carbon, stir and moisten, set aside; inject water for injection with a dilute dosage of 20%, Add a pH regulator, stir to dissolve, adjust the water temperature to ≤30°C, then add adenosine monophosphate, stir until completely dissolved, add water for injection to 70% of the total amount, and the pH of the liquid to 7.0-7.5; add active Carbon, heated to 80°C, stirred and adsorbed for 15 minutes; 0.22μm sterilized filter, sterilized with pure steam at 121°C, 20 minutes on-line; supplemented with water for injection to 95% of the theoretical preparation amount, took samples to measure the content, and calculated according to the content. Water for injection to 2000ml, at the same time turn on the stirring and liquid return pump circulation for not less than 10 minutes, take...
Embodiment 2
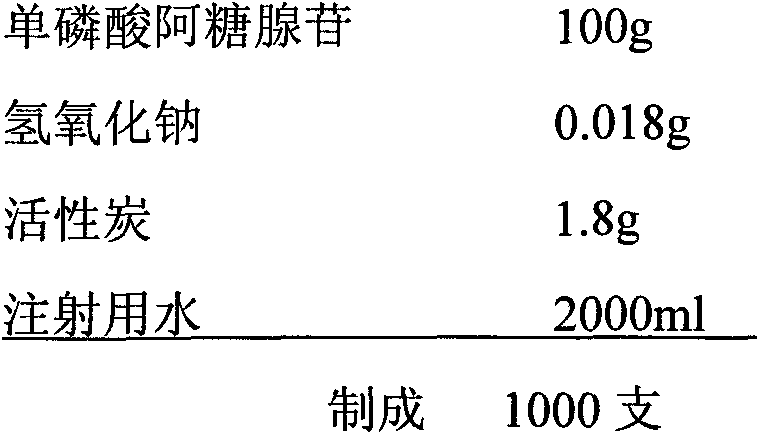
[0026]
[0027] Weigh 100g of vidarabine monophosphate and 0.018g of sodium hydroxide respectively, and set aside; weigh 1.8g of activated carbon, add water for injection about 3 times the amount of activated carbon, stir and moisten, set aside; inject water for injection with a dilute dosage of 25%, Add a pH regulator, stir to dissolve, adjust the water temperature to ≤30°C, then add adenosine monophosphate, stir until completely dissolved, add water for injection to 70% of the total amount, and the pH of the liquid to 7.0-7.5; add active Carbon, heated to 80°C, stirred and adsorbed for 15 minutes; 0.22μm sterilized filter, sterilized with pure steam at 121°C, 20 minutes on-line; supplemented with water for injection to 95% of the theoretical preparation amount, took samples to measure the content, and calculated according to the content. Water for injection to 2000ml, at the same time turn on the stirring and liquid return pump circulation for not less than 10 minutes, tak...
Embodiment 3
[0029]
[0030] Weigh 50 g of vidarabine monophosphate, 0.029 g of disodium hydrogen phosphate, and 0.001 g of sodium dihydrogen phosphate, and set aside; weigh 1.5 g of activated carbon, add water for injection about 3 times the amount of activated carbon, stir and moisten, set aside; inject dilute Dosing 30% water for injection, adding a pH regulator, stirring to dissolve, adjusting the water temperature to ≤30°C, then adding adenosine monophosphate, stirring until completely dissolved, supplementing water for injection to 70% of the total amount, and pH of the liquid medicine value to 7.0-7.5; add activated carbon, heat to 80°C, stir and adsorb for 15 minutes; sterilize and filter at 0.22μm, use pure steam at 121°C, and sterilize on-line for 20 minutes; add water for injection to 95% of the theoretical preparation amount, and take samples Measure the content, calculate and add water for injection to 2000ml according to the content, and at the same time turn on the stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com