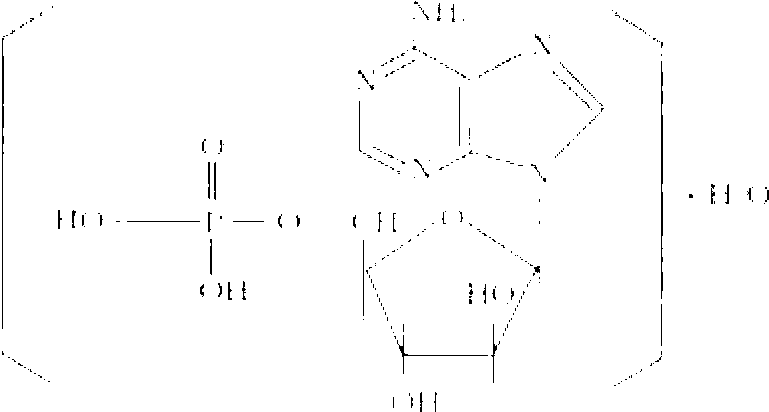
Vidarabine monophosphate pharmaceutical composition and preparation method thereof
A technology of adenosine vidarabine monophosphate and adenosine vidarabine, which is applied in the field of medicine, can solve problems such as stimulation, and achieve the effects of reducing pain, good stability, and high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] [Example 1] Preparation of adenosine monophosphate monophosphate composition for injection
[0048] Prescription: specification 0.1g (calculated as adenosine vidarabine monophosphate)
[0049]
[0050] Preparation:
[0051] (1) Put 500g of hydroxypropyl β-cyclodextrin in 15kg of water for injection, stir at 200rp / min at 60°C for 2h to dissolve the hydroxypropyl β-cyclodextrin, then cool to 30°C, Slowly add 66.7 g of vidarabine under stirring for 1 min to dissolve to obtain solution A;
[0052] (2) Add vidarabine monophosphate and sodium chloride into solution A, stir to dissolve, and obtain solution B;
[0053] (3) Add 79g of activated carbon for needles to the above solution B, keep at 30°C, stir at 400rp / min for 35min, filter through a 4μm water-based filter membrane to remove the needle-use activated carbon, then filter through a 0.2μm water-based filter membrane, and sterilize to obtain sterile filtrate;
[0054] (4) Adjust the filling volume in the center of...
Embodiment 2
[0060] [Example 2] Preparation of adenosine monophosphate and sulfobutyl ether β-cyclodextrin composition for injection
[0061] Prescription: specification 0.1g (calculated as adenosine vidarabine monophosphate)
[0062]
[0063] Preparation:
[0064] (1) Put 400g of sulfobutyl ether β-cyclodextrin in 16kg of water for injection, stir at 200rp / min at 70°C for 2h to dissolve the sulfobutyl ether β-cyclodextrin, then cool to 40°C, Slowly add 40 g of vidarabine under stirring at 500rp / min to dissolve to obtain solution A;
[0065] (2) Add vidarabine monophosphate and sodium chloride into solution A, stir to dissolve, and obtain solution B;
[0066] (3) Add 79g of activated carbon for needles to the above solution B, keep at 40°C, stir at 500rp / min for 25min, filter through a 4μm water-based filter membrane to remove the needle-use activated carbon, then filter through a 0.2μm water-based filter membrane, and sterilize to obtain sterile filtrate;
[0067] (4) Adjust the fi...
Embodiment 3
[0073] [Example 3] Preparation of vidarabine monophosphate for injection
[0074] Prescription: specification 0.1g (calculated as adenosine vidarabine monophosphate)
[0075]
[0076] Preparation:
[0077] (1) Put 572g of carboxymethyl β-cyclodextrin in 16kg of water for injection, stir at 200rp / min at 65°C for 2h to dissolve carboxymethyl β-cyclodextrin, then cool to 35°C, Slowly add 85.8 g of vidarabine under stirring for 1 min to dissolve to obtain solution A;
[0078] (2) Add vidarabine monophosphate and sodium chloride into solution A, stir to dissolve, and obtain solution B;
[0079] (3) Add 79g of activated carbon for needles to the above solution B, keep at 35°C, stir at 450rp / min for 30min, filter through a 4μm water-based filter membrane to remove the needle-use activated carbon, then filter through a 0.2μm water-based filter membrane, and sterilize to obtain sterile filtrate;
[0080] (4) Adjust the filling capacity in the center of the filling machine to 2.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com