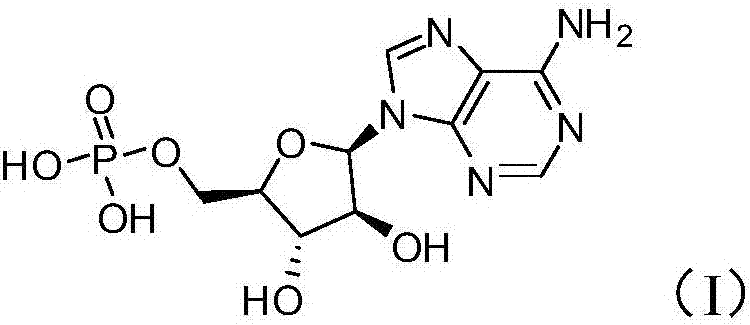
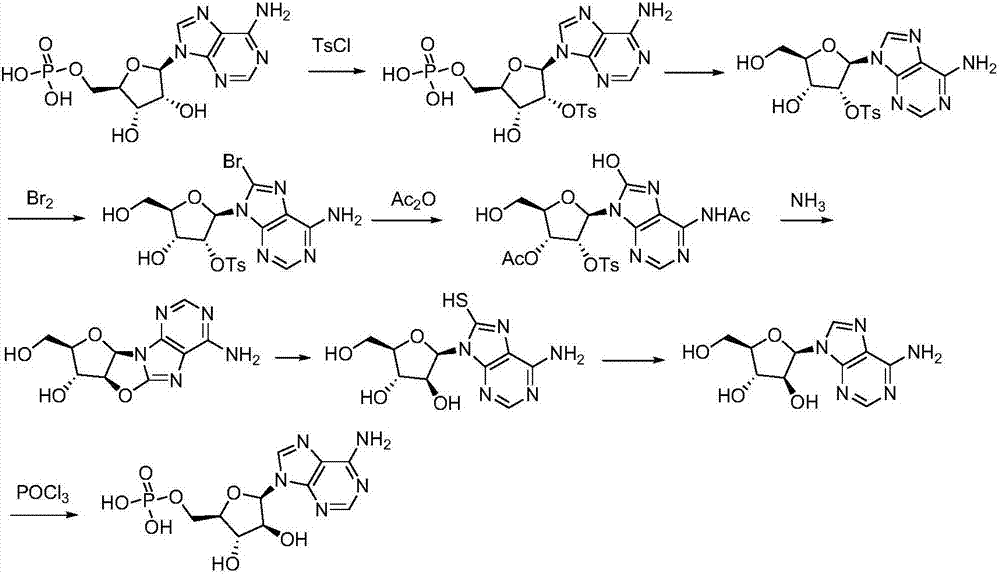
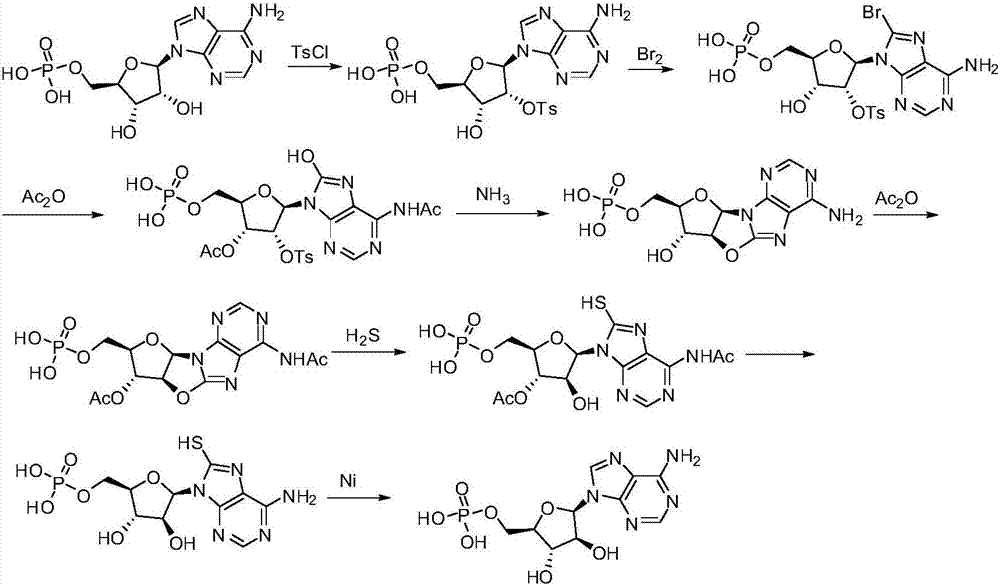
Synthetic method of vidarabine monophosphate
A technology of vidarabine monophosphate and synthesis method, which is applied in the field of medicine, can solve problems such as high environmental pressure, and achieve the effects of alleviating environmental pressure, alleviating odor problems, and alleviating environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: the synthesis of intermediate 1
[0059]
[0060] Adenosine monophosphate (348g, 1.0mol) was added to 1.5L 1,4-dioxane, stirred for 10 minutes, the resulting solution was cooled to below 0-10°C, and 3.5L aqueous sodium hydroxide solution (1.0mol / L), control system temperature 5~15 ℃. The resulting mixed solution continued to stir for 30 minutes, cooled to below -15 to 0°C, and slowly added dropwise a 1,4-dioxane solution of p-toluenesulfonyl chloride (229g of toluenesulfonyl chloride dissolved in 1.0L of dioxane), During the process, the temperature of the reaction system was controlled at 0-5° C., and the stirring was continued for 18 hours after the dropwise addition was completed.
[0061] Concentrate the above solution to 4 L in a concentrator not higher than 30 degrees Celsius, add dilute hydrochloric acid to adjust the pH value to 4.0, stir well, leave it at room temperature overnight, and collect the solid by filtration to obtain 489 g of Inter...
Embodiment 2
[0062] Embodiment 2: the synthesis of intermediate 2
[0063]
[0064] Intermediate 1 (485g, 0.95mol) was added to 2L 1,4-dioxane, stirred and dissolved, the system was cooled to -15 degrees Celsius, and solid pyridinium bromide (330g, 1.03mmol, 1.08equ. ) slowly added to the system in batches, keeping the reaction temperature of the system within the range of 0-5°C.
[0065] After the addition, keep the temperature of the reaction system within the range of 0-5°C, continue to stir for 0.5 hours, add water to dilute the reaction solution to 4L, adjust the pH value to 4.0, concentrate the system volume to 3L, let it stand overnight, and collect the solid by filtration to obtain 503.1g intermediate Body 2, yield 91.2%.
Embodiment 3
[0066] Embodiment 3: the synthesis of intermediate 3
[0067]
[0068] Intermediate 2 (500g) was added into a mixed solution composed of 800 milliliters of acetic acid and 400 milliliters of acetic anhydride, the system was heated to reflux, kept stirring for 2 hours, added 400 milliliters of methanol, continued to stir for 0.5 hours, concentrated to dryness, and added ethanol ( 800 ml) was concentrated to dryness, and another 800 ml of ethanol was added to concentrate to dryness, and the obtained residue was recrystallized with water to obtain 463.7 g of solids, with a yield of 89.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com