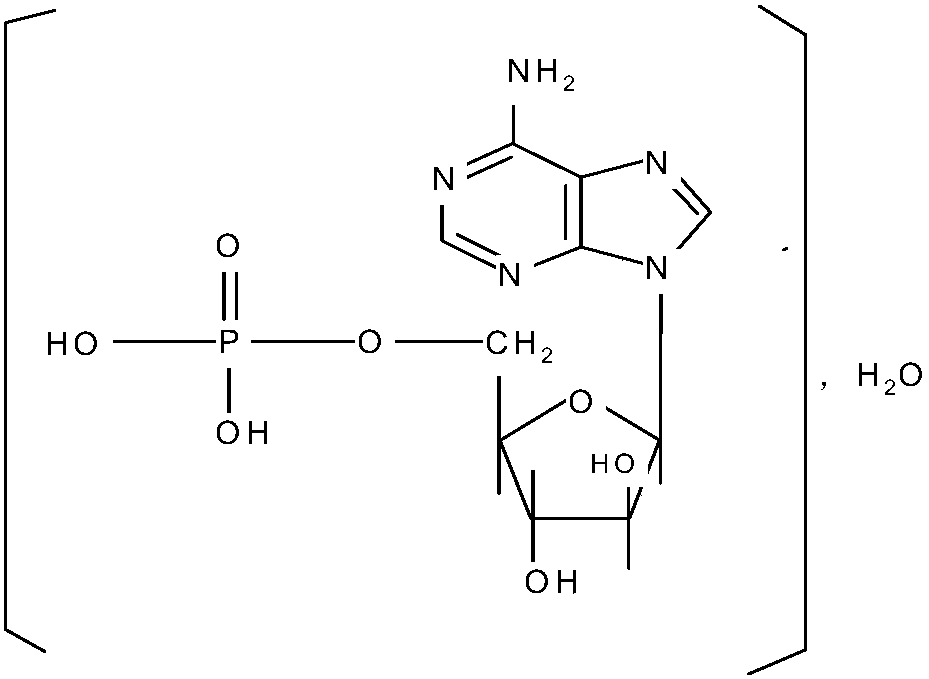
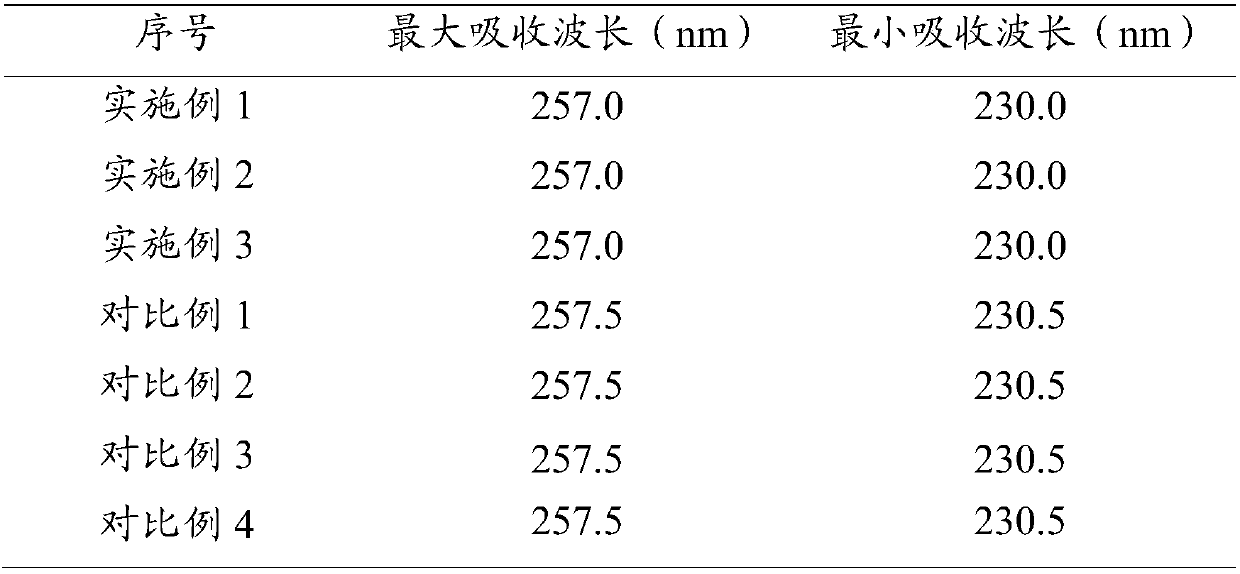
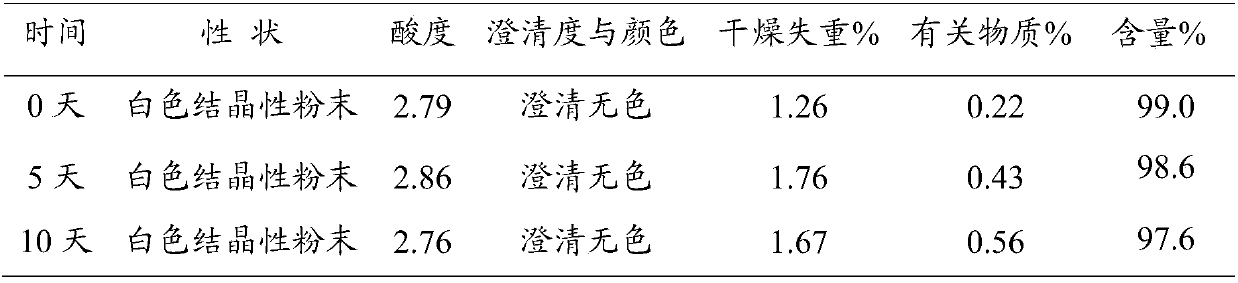
Preparation method of vidarabine monophasphate
A vidarabine monophosphate and vidarabine monophosphate wet crude product technology, which is applied in the field of preparation of vidarabine monophosphate, can solve problems such as complex process operation, affecting product yield and final product quality, and achieve operational Simple process, low cost, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of Crude Arabinosine Monophosphate:
[0032] 1. Prepare an aqueous sodium hydroxide solution with a mass fraction of 40%: add 37.5kg of purified water to a 50L reaction kettle, add 15.0kg of sodium hydroxide in portions under stirring, and stir to dissolve for later use.
[0033] 2. Add 10.0 kg of β-D-arabinoid adenosine and 100.0 kg of triethyl phosphate into a 200L reaction tank, and cool to -10°C.
[0034] 3. Add 10.2 kg of phosphorus oxychloride dropwise under stirring, control the temperature not to be higher than 0°C, and complete the dropwise addition within 3 to 5 hours; after the dropwise addition is completed, stir and react at -5°C for 90 minutes, and the TLC detection reaction reaches the end point, stop reaction.
[0035] 4. Slowly pump the reaction solution into a 500L reaction tank (filled with 150kg of purified water pre-frozen to 0°C), and control the temperature not to be higher than 15°C.
[0036] 5. Cool down to 0°C, add dropwise a 40% NaOH aqueous ...
Embodiment 2
[0049] Synthesis of Crude Arabinosine Monophosphate:
[0050] 1. Prepare an aqueous sodium hydroxide solution with a mass fraction of 40%: add 37.5kg of purified water to a 50L reaction kettle, add 15.0kg of sodium hydroxide in portions under stirring, and stir to dissolve for later use.
[0051] 2. Add 10.0 kg of β-D-arabinoid adenosine and 100.0 kg of triethyl phosphate into a 200L reaction tank, and cool to -13°C.
[0052] 3. Add 10.2 kg of phosphorus oxychloride dropwise with stirring, control the temperature not to be higher than 0°C, and complete the dropwise addition within 3 to 5 hours; after the dropwise addition is completed, stir and react at -2.5°C for 120 minutes, the TLC detection reaction reaches the end point, stop reaction.
[0053] 4. Slowly pump the reaction liquid into a 500L reaction tank (filled with 150kg of purified water pre-frozen to 1.5°C), and control the temperature not to be higher than 15°C.
[0054] 5. Cool down to 0°C, add dropwise a 40% NaOH aqueous so...
Embodiment 3
[0067] Synthesis of Crude Arabinosine Monophosphate:
[0068] 1. Prepare an aqueous sodium hydroxide solution with a mass fraction of 40%: add 37.5kg of purified water to a 50L reaction kettle, add 15.0kg of sodium hydroxide in portions under stirring, and stir to dissolve for later use.
[0069] 2. Add 10.0kg of β-D-arabinoid adenosine and 100.0kg of triethyl phosphate into a 200L reaction tank, and cool down to -15℃.
[0070] 3. Add 10.2kg phosphorus oxychloride dropwise with stirring, control the temperature not to be higher than 0℃, and complete the dripping within 3 to 5 hours; after the dripping is completed, stir and react at -0℃ for 150min, TLC detection reaction reaches the end point, stop reaction. Add 10.2kg phosphorus oxychloride dropwise with stirring, and control the temperature not to be higher than 0℃.
[0071] 4. Slowly pump the reaction liquid into a 500L reaction tank (containing 150kg of purified water pre-frozen to 2°C), and control the temperature to not higher ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com