Vidarabine monophosphate microsphere drug delivery system and preparation method thereof
A technology of adenosine monophosphate and chitosan microspheres, which is applied in the field of medicine, can solve the problems of low patient compliance and short half-life, and achieve the effects of stable release, good dispersion, and good sustained release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Weigh 0.4g of chitosan (deacetylation degree 96%) and dissolve in 20mL of 2% acetic acid aqueous solution, let it stand, and remove air bubbles. Measure 0.1 g of polyethylene glycol 400 and 0.1 g of vidarabine monophosphate and add to the above solution to form the water phase. Take 100mL of liquid paraffin, add 5mL of Tween 80 and 0.5g of magnesium stearate as the oil phase. Slowly pour the water phase into the oil phase, mechanically stir at 1000rpm, add 2mL glutaraldehyde after complete emulsification, continue stirring for 4h to cross-link and solidify, collect the precipitate, wash with isopropanol and petroleum ether, and dry.
[0029] Under the electron microscope, it can be seen that the microspheres are round and spherical, and the results are shown in figure 1 .
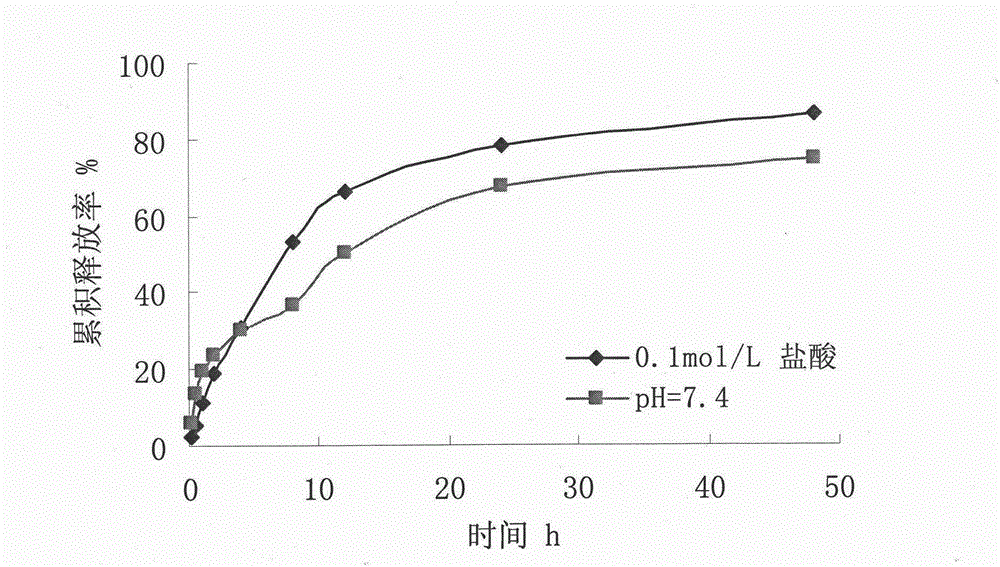
[0030] Destroy the microspheres with 0.25mol / L hydrochloric acid, measure the content of vidarabine monophosphate in the microspheres by HPLC, and calculate the encapsulation efficiency according t...
Embodiment 2
[0036] Weigh 0.6g of chitosan (degree of deacetylation 96%) and dissolve in 20mL of 2% acetic acid aqueous solution, let stand, and remove air bubbles. Measure 0.2g of polyethylene glycol 400 and 0.06g of vidarabine monophosphate into the above solution to form the water phase. Take 60mL of liquid paraffin, add 2mL of Tween 80 and 0.2g of magnesium stearate as the oil phase. Slowly pour the water phase into the oil phase, stir mechanically at 1200rpm, add 1mL glutaraldehyde after complete emulsification, continue stirring for 6h to cross-link and solidify, collect the precipitate, wash with isopropanol and petroleum ether, and dry.
[0037] The encapsulation efficiency of the microspheres was calculated to be 76%.
Embodiment 3
[0039] Weigh 0.1 g of chitosan (deacetylation degree 96%) and dissolve in 20 mL of 2% acetic acid aqueous solution, let stand, and remove air bubbles. Measure 0.02g of polyethylene glycol 4000 and 0.1g of vidarabine monophosphate and add to the above solution to form the water phase. Take 40mL of liquid paraffin, add 1mL of Tween 80 and 0.1g of magnesium stearate as the oil phase. Slowly pour the water phase into the oil phase, stir mechanically at 1200rpm, add 0.2mL glutaraldehyde after the emulsification is complete, continue stirring for 2h to cross-link and solidify, collect the precipitate, wash with isopropanol and petroleum ether, and dry.
[0040] The encapsulation efficiency of the microspheres was calculated to be 37%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com