Globulin pasteurization process using combined protective agent
A globulin and protective agent technology, applied in the field of human immunoglobulin inactivation, can solve problems such as difficulty in ensuring protein yield and quality at the same time, achieve the effects of reducing process troubles, simple and effective verification, and low equipment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0040] The production process of human immunoglobulin includes: combined plasma, fraction I precipitation separation, fraction II+III precipitation separation, fraction III precipitation separation, fraction II precipitation separation, fraction II precipitation dissolution and ultrafiltration, pasteurization Inactivation, purification and preparation of aliquots. This protocol mainly adds a protective agent in the pasteurization step to ensure the yield and content of the target protein.
[0041] 1. Raw plasma treatment: After the raw plasma leaves the warehouse, use 70-75vol.% ethanol solution to sterilize the surface of the plasma bag, then break the plasma bag, control the temperature to melt at 0-4°C, combine the plasma after melting, and use a centrifuge Centrifuge plasma (centrifugal force: 10000RCF), collect supernatant A. Among them, the raw plasma is the supernatant after blood centrifugation to remove cells, which contains protein, inorganic salts and water, etc., ...
experiment example 1
[0050] Experimental Example 1: Study on a single protective agent
[0051] Obtain the filtrate after the ultrafiltration of equal volume through the above-mentioned steps 1-6, and the specific operation steps are:
[0052] 1. Raw material plasma treatment: After the raw material plasma leaves the warehouse, use 75Vol.% ethanol solution to disinfect the surface of the plasma bag, then break the plasma bag, control the temperature to melt at 0°C, combine the plasma after melting, and use a centrifuge to centrifuge the plasma ( The centrifugal force is 10000RCF), and the supernatant A is collected.
[0053] 2. Precipitation and separation of component I: adjust the temperature of the supernatant A to -3.0°C, the protein concentration to 65g / L, the pH to 7.30, the conductivity to 14mS / cm, and the volume percent concentration of ethanol (pure ethanol) to 10vol. % (final concentration), reacted for 3 hours, and collected the supernatant B by pressure filtration.
[0054] 3. Precip...
experiment example 2
[0062] Experimental Example 2: Research on Protective Agent Combination
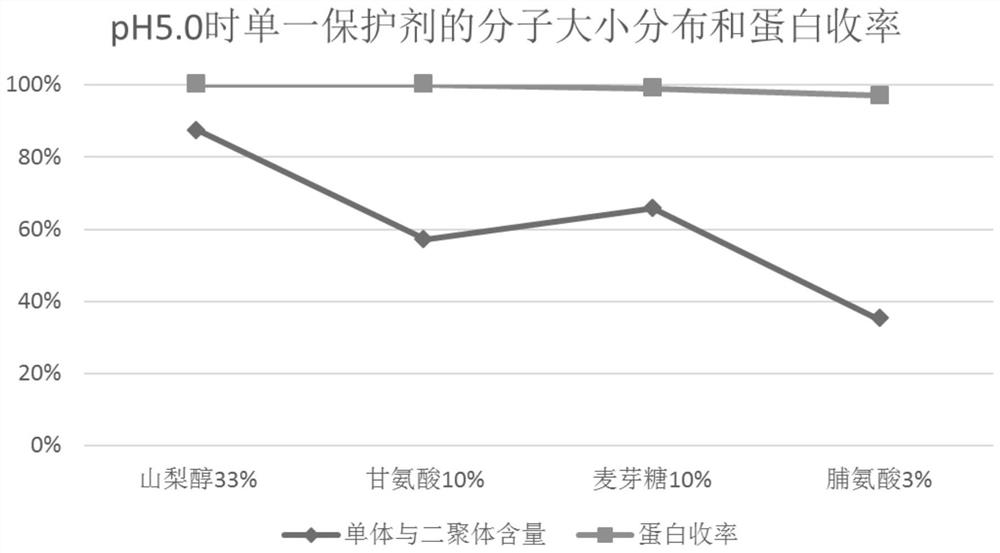
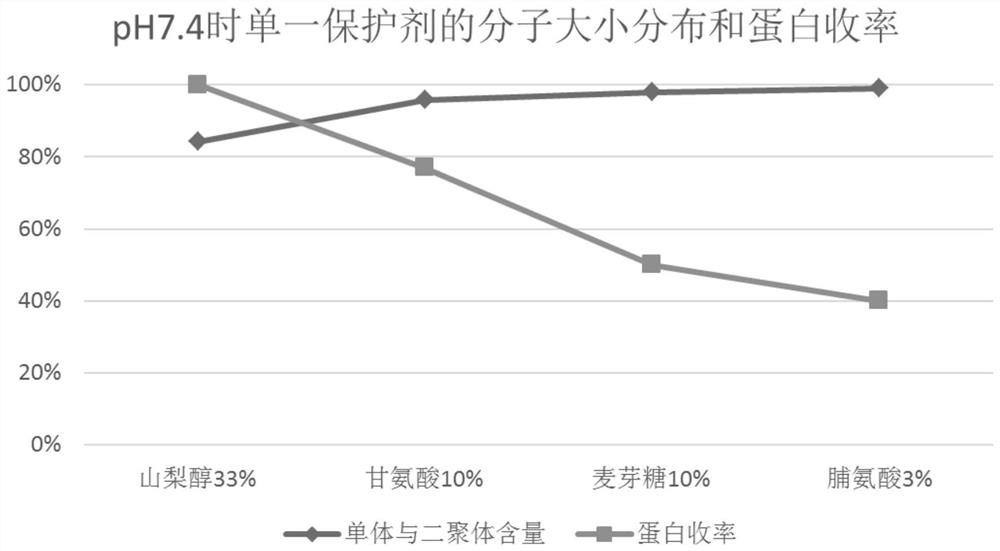
[0063] According to the method of Experimental Example 1, the filtrate after ultrafiltration of equal volume (here may be referred to as protein solution) was prepared, and the protein solution was divided into 6 parts on average, and sorbitol, glycine, and maltose were used to combine in pairs, and then adjusted with 1.0 mol / l citric acid or 0.5mol / l NaOH was used to adjust the pH to 5.0±0.05 and 7.4±0.05, and water for injection was added until the protein content was 20g / l. Pasteurization was carried out after filtering with a terminal 0.22 μm filter element. The inactivation conditions were 60±0.5°C, constant temperature for 10 hours, and cooled to room temperature after constant temperature. The inactivated product is tested, and the test results are shown in Table 2, Figure 4 , Figure 5 and Figure 6 . exist figure 1 The treatment condition of 1 is sorbitol 33wt.%+glycine 10wt.%+pH 5.0; the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com