Application of pazopanib, pharmaceutical composition, injection, preparation method and application
A technology of pazopanib and its composition, applied in the fields of application, pharmaceutical composition, injection and preparation and application of pazopanib, capable of solving the problems of no introduction of acute lung injury, no acute lung injury, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
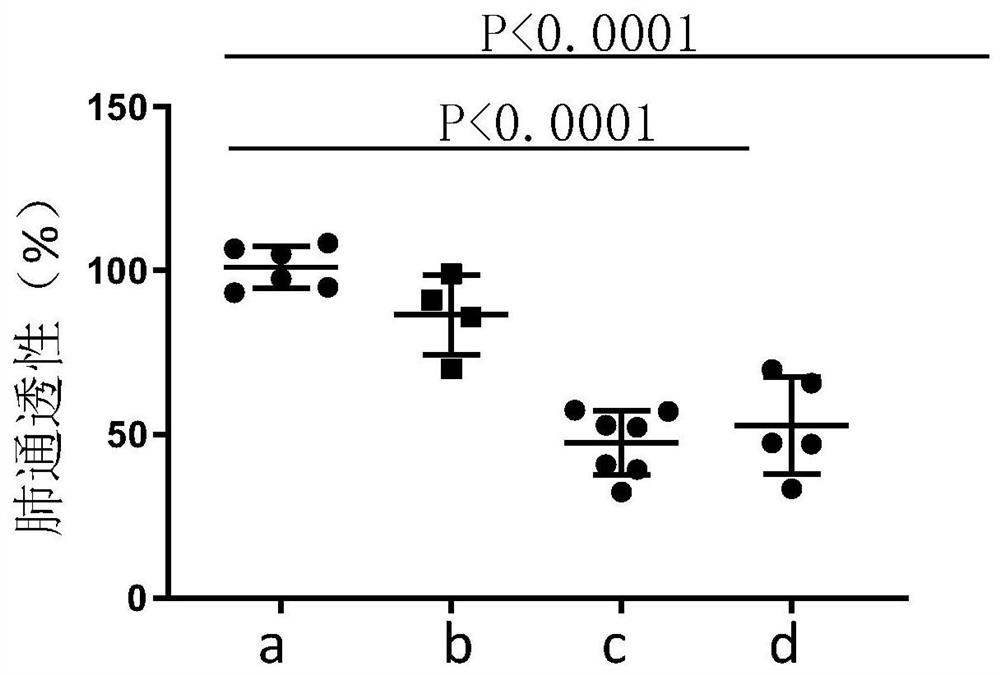
Image
Examples
Embodiment 1
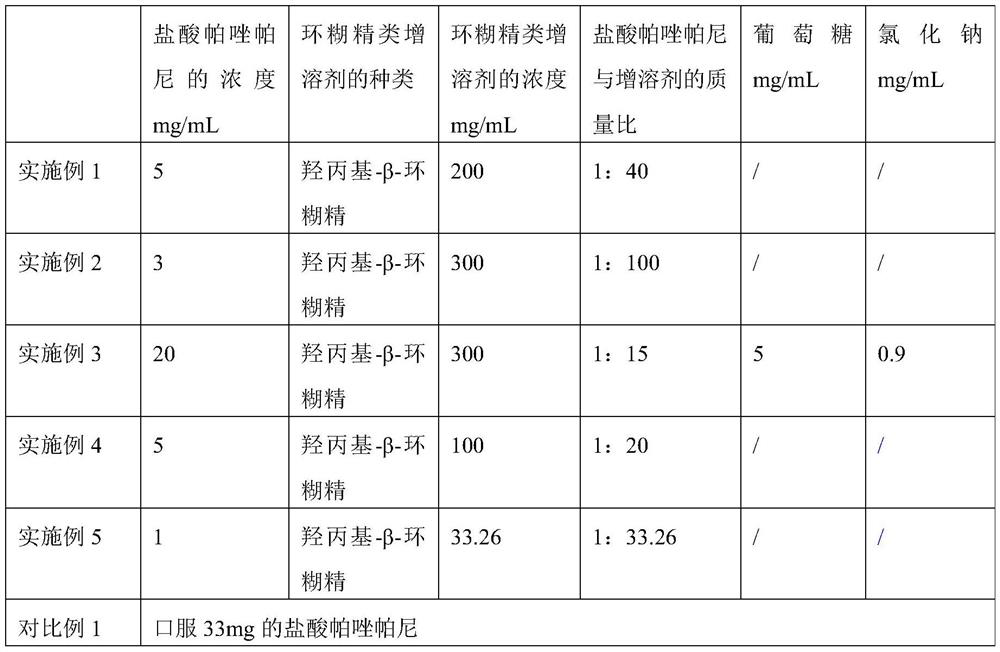
[0065] Embodiment 1 pharmaceutical composition and preparation method thereof
[0066] 1. Taking the pharmaceutical composition as 1 mL; the concentration of pazopanib hydrochloride in the pharmaceutical composition is 5 mg / mL, and the concentration of hydroxypropyl-β-cyclodextrin in the pharmaceutical composition is 200 mg / mL; the concentration of the pharmaceutical composition The pH is 3.25.
[0067] 2. The preparation method of the above-mentioned pharmaceutical composition specifically comprises the following steps:
[0068] (1) Pour 5 mg of pazopanib hydrochloride into a container containing 1 mL of 200 mg / mL hydroxypropyl-β-cyclodextrin solution, the solvent in the hydroxypropyl-β-cyclodextrin solution For injection water. The concentration of pazopanib hydrochloride in the mixed solution is 5 mg / mL, and the concentration of hydroxypropyl-β-cyclodextrin is 200 mg / mL; among them, the water for injection needs to be filled with nitrogen for 30 minutes before use;
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com