Solution agent for recombining human interleukin 12 and preparation method thereof
A technology of interleukin and solution, which is applied in the direction of non-active ingredient medical preparations, active ingredient-containing medical preparations, antiviral agents, etc., and can solve the problems that satisfactory rhIL-12 liquid injections have not yet been developed. , to achieve the effect of good appearance, good stability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] According to pH = 6, add phosphate buffer solution PB, add appropriate amount of water to dissolve, then add NaCl to make the final concentration 150mM, after dissolving, add poloxamer 188 with a final concentration of 0.1%, after completely dissolving, wash with 1M NaOH Adjust the pH to 6.0, and add recombinant human interleukin-12 at a final concentration of 10 μg / ml to prepare the solution of the present invention. It is given as Formulation 4 in the formulation formulation screening experiments described below.
[0026] The specific preparation, components and advantages of the solution of the present invention will be described below through the formulation screening experiment.
Embodiment 2
[0028] 1. Preliminary screening of dosage forms
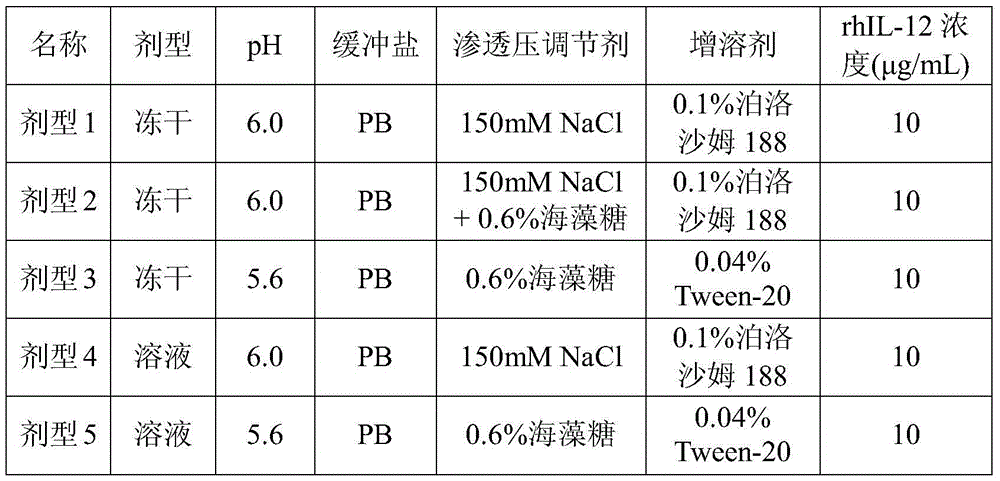
[0029] 1.1 Dosage Form Design
[0030] Table 1 dosage form design scheme
[0031]
[0032] The dosage form design considers both lyophilized powder and solution. The lyophilized powder is easy to store and transport, but the lyophilization process is more complicated. Therefore, both types of preparations are included in the scope of investigation.
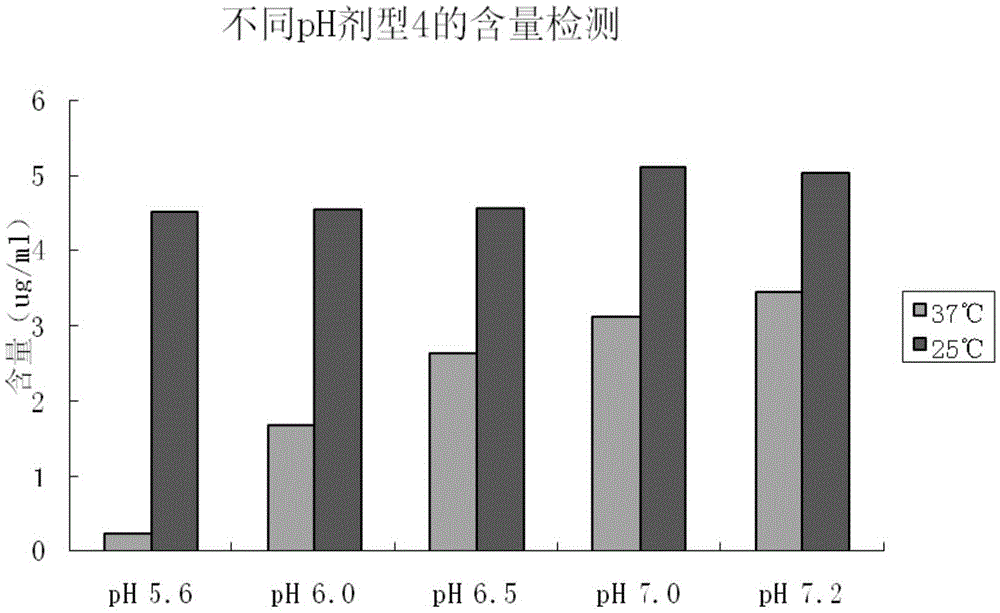
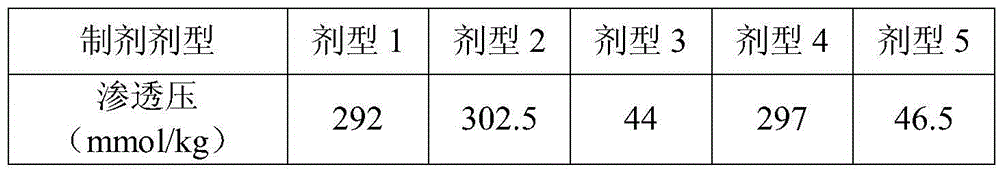
[0033] In the initial experiment, 3 lyophilized powder and 2 solution dosage forms were designed, totaling 5 dosage forms. Detect and analyze from several aspects such as appearance, content, activity, etc., compare the stability of various dosage forms under certain conditions (25°C), and screen better dosage forms.
[0034] 1.2 Dosage Form Screening Experiment
[0035] 1.2.1 Visual inspection
[0036] Dosage forms 1, 2, and 3 are freeze-dried powders, and their appearance is as follows:
[0037] Dosage Form No. 1: most powder cakes are complete, without loose lumps or pow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com