Method for inducing mammary epithelial cell differentiation
A technology of mammary epithelial cells and mammary gland tissue, applied in the field of inducing the differentiation of mammary epithelial cells, increasing the milk production of lactating mammals, and enhancing the development of mammalian mammary glands, which can solve the problem of low sequence similarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
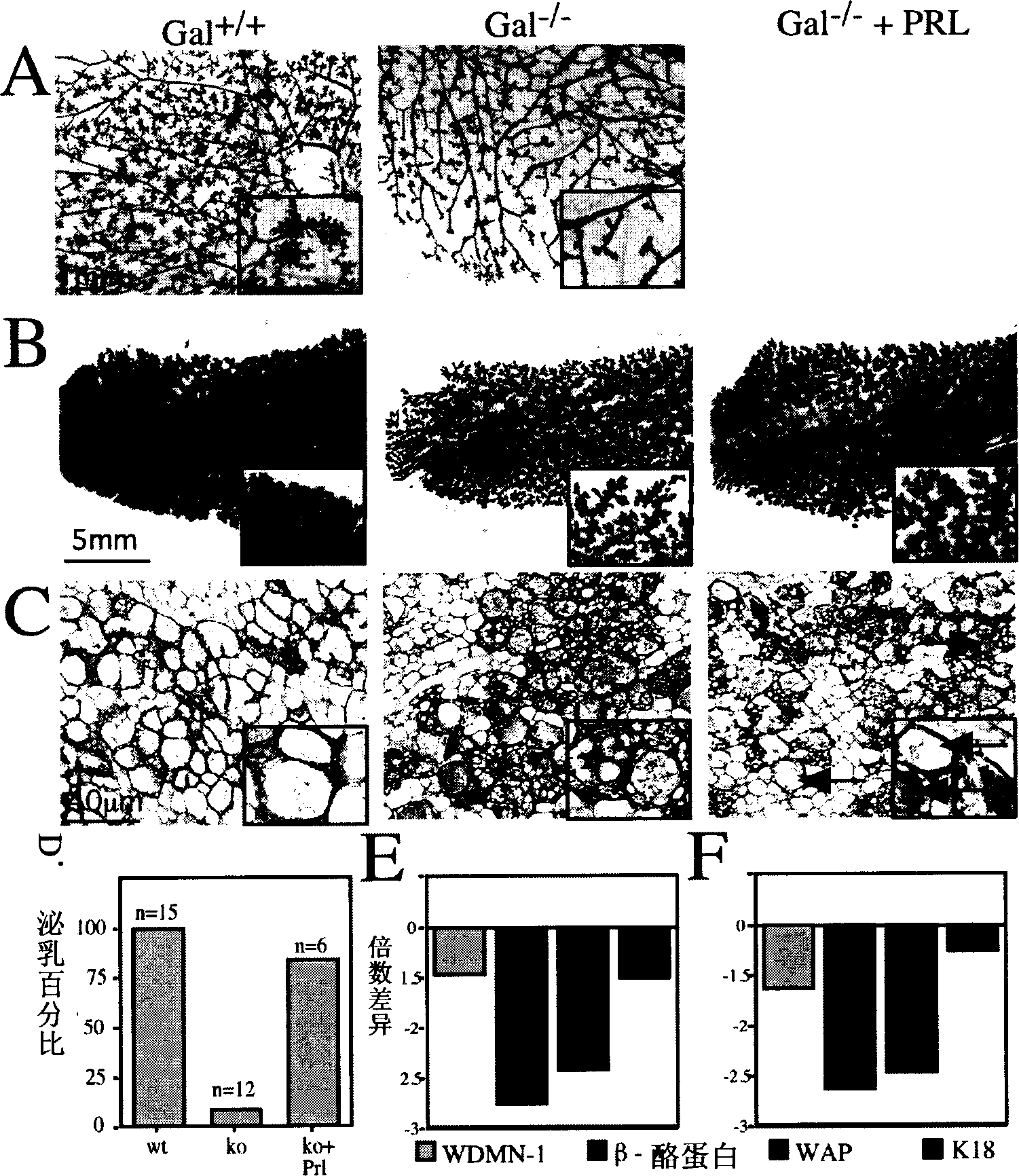
[0204] Embodiment 1: to Gal - / - Supplementation of prolactin in mice induced lactation sufficient for pup survival, but lobular alveolar development was not fully restored.
[0205] Targeted disruption of the galanin gene causes ductal lateral branch defects in puberty and impaired lactation in first pregnancy. We have previously attributed this effect to Gal - / - Reduced serum prolactin levels were seen in animals, but this hypothesis was not tested, nor were defects in the Gal+ / + mammary glands studied during pregnancy (17). On day 12 of gestation, wild-type galanin (Gal + / + ) mice compared to ( figure 1 A), Gal - / - Reduced size and density of lobular alveoli in the mammary gland. This defect persists throughout pregnancy and the first postpartum day, with Gal + / + mice ( figure 1 B) compared to Gal - / - The mammary glands showed decreased lobular alveolar density. Histological examination revealed that despite lobular vesicle formation, Gal - / - Lactation did not star...
Embodiment 2
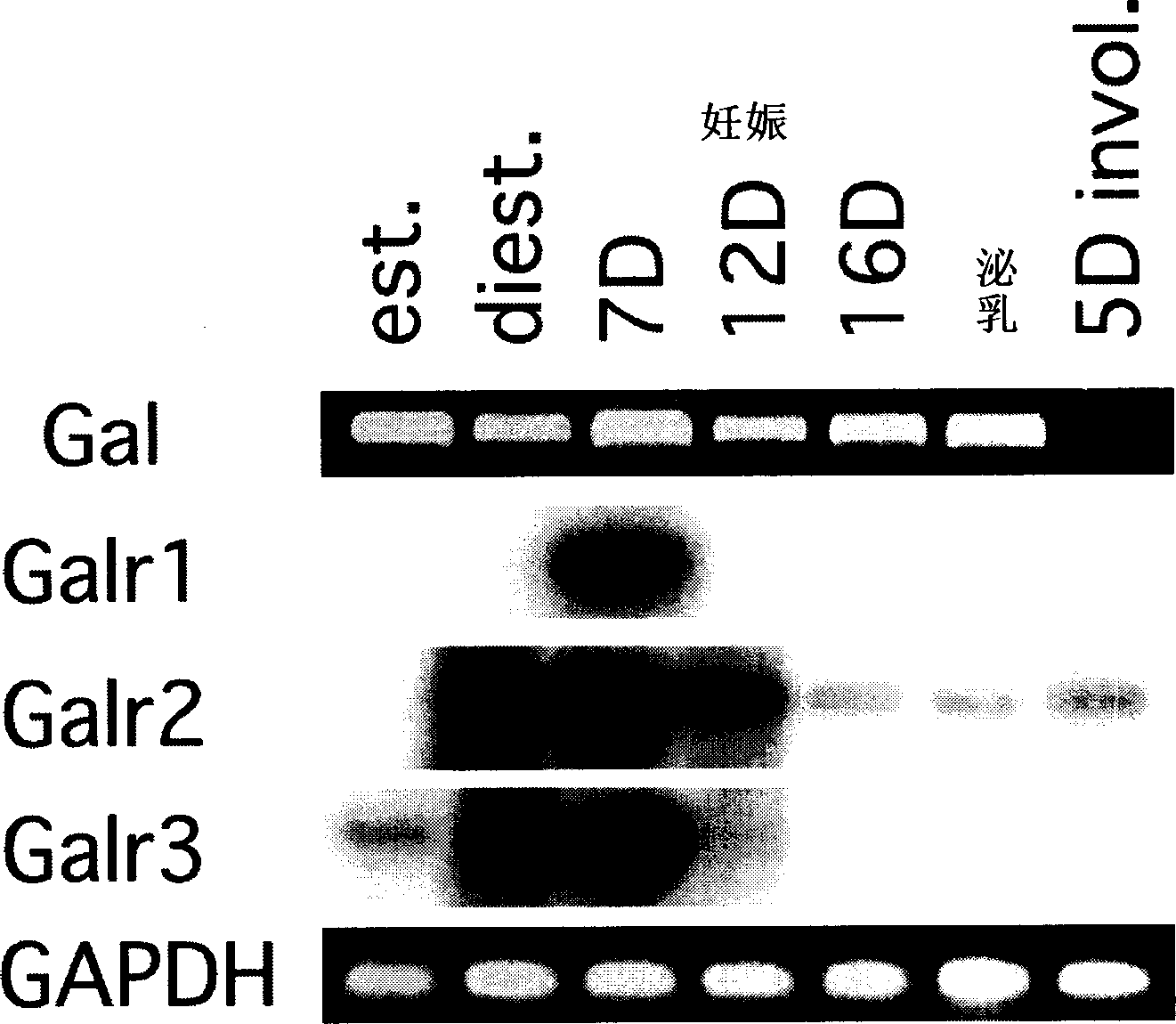
[0207] Example 2: Galanin and galanin receptors are differentially expressed in the mammary gland.
[0208] Gal treated with PRL - / - The inability of mice to restore milk protein expression suggests that galanin may act through an additional mechanism to regulate mammary epithelial differentiation. The mRNAs expressing galanin and Galr1-3 were examined by RT-PCR using mouse mammary glands collected at different developmental stages ( figure 2 ). Galanin transcripts were expressed at all time points, from estrus to lactation in nulliparous mice, but were not detected during mobilization. Expression of galanin receptor transcripts is tightly regulated or coordinated. Transcript expression for all three receptors was highest on day 7 of gestation. Galr1 transcripts were only detectable at this time, whereas Galr2 mRNA was also detectable at lower levels during late gestation and mobilization, and Galr3 mRNA was also detectable during estrus and anestrus in nulliparous mice ...
Embodiment 3
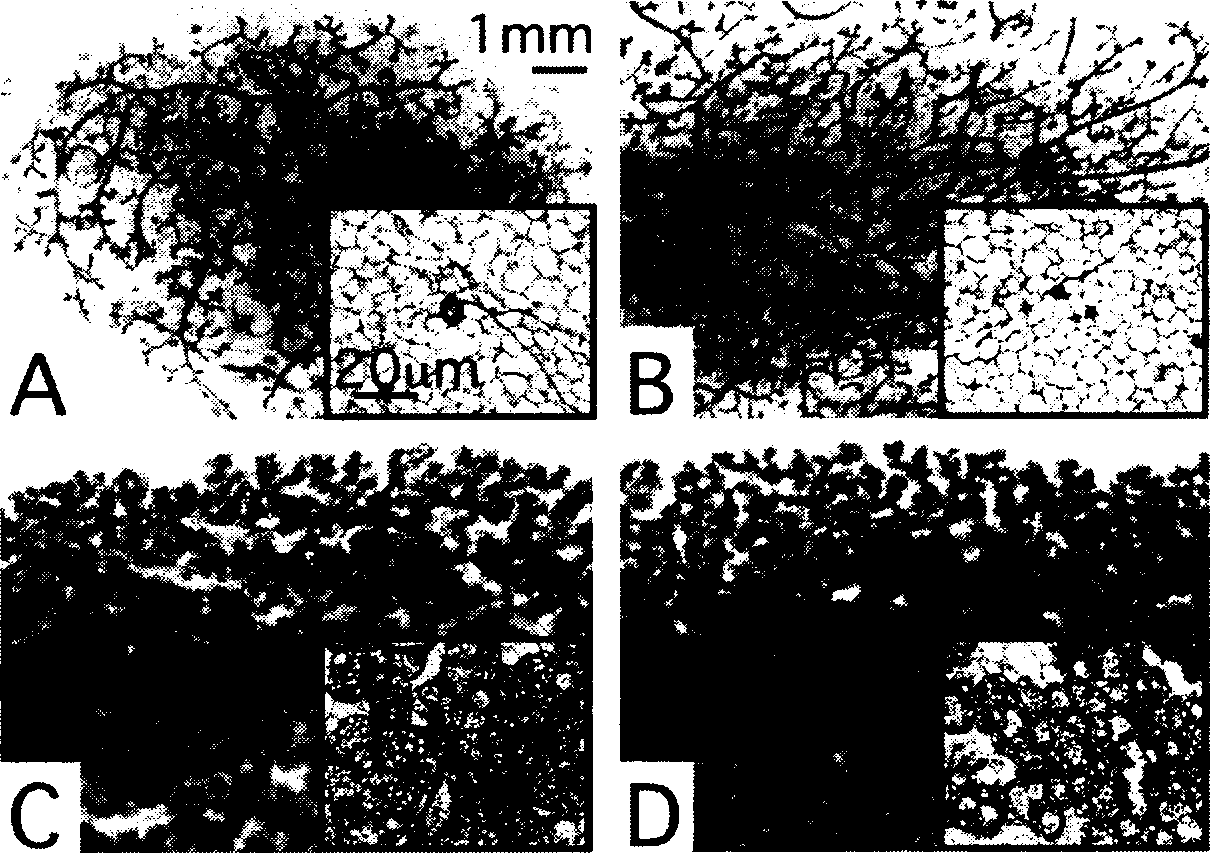
[0210] Example 3: The autocrine or paracrine mechanism of action of galanin is not required for mammary gland development.
[0211] To determine whether galanin produced by the mammary gland is required for normal development, we used mammary epithelial transplantation. From Mature Gal + / + or Gal - / - Donor mammary epithelium from mice was transplanted into 3-week-old Gal + / + or Gal - / - in the excised fat pad of mice. Reconstituted mammary epithelial-stromal complexes were inoculated in 3-week-old Ragl - / - between the skin and abdominal cavity of mice (22). This allows removal of the galanin gene from the stroma and / or epithelium under normal endocrine conditions including normal circulating prolactin and galanin levels.
[0212] Removal of galanin from stroma, epithelium, or both does not restore unproduced Gal - / - The impairment of ductal lateral branching observed in mice also failed to restore the impaired lobuloalveolar development seen in the first postnatal day ( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com