Dextrooracetam freeze-dried powder injection with good stability and preparation method thereof
A technology of freeze-dried powder injection and freeze-dried excipients, which is applied in the field of dexoxiracetam preparations, can solve the problems of unstable quality, high related substances, and high water content of freeze-dried preparations, and achieve good clarity, Good resolubility, considerable economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
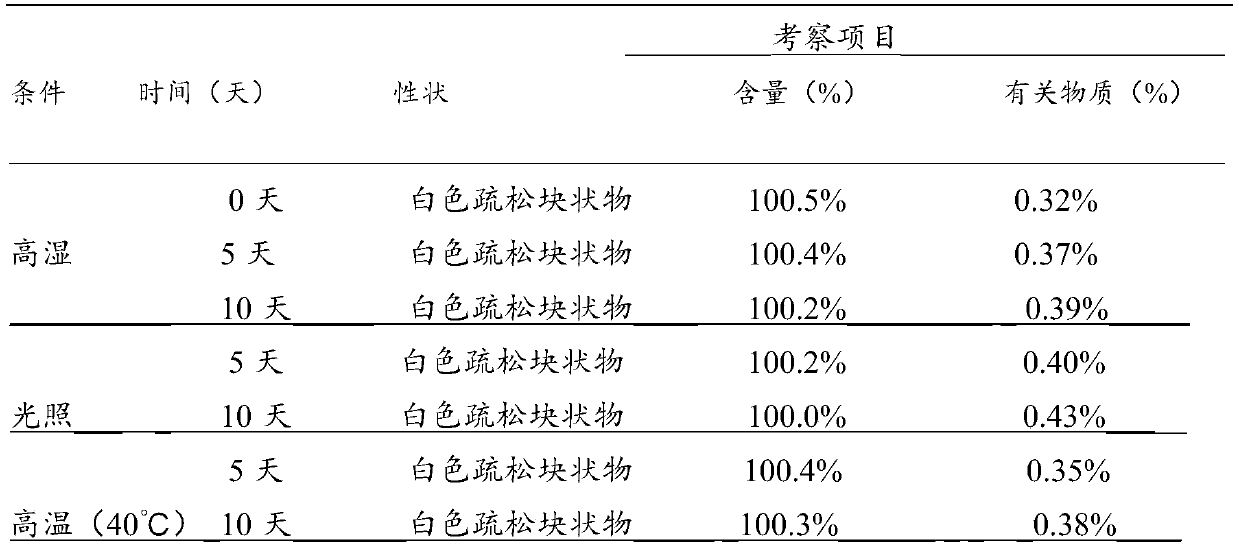
[0029]Purification of Dexoxiracetam: In 150mL of methanol, add 10g of Dexoxiracetam, stir continuously, heat to 50°C, dissolve completely, add 0.5g of activated carbon and stir for 30 minutes at a speed of 250r / min, filter , to obtain a methanol solution, the methanol solution was concentrated to a total volume of 20ml to obtain a saturated methanol solution, adding 30ml of ethanol, stirring and crystallizing at a speed of 250r / min in a 5°C environment, and filtering to obtain colorless sandy crystals. Dry at -70°C for 4-5 hours at a relative humidity of 20-25%, and collect crystals.
[0030] Powder Diffraction Determination (XRPD): Test Instrument Conditions: Use Bruker D2PHASER powder diffractometer to carry out normal temperature test, test conditions are: with Cu Ka It is the light source, the voltage is 30kV, the current is 10mA, the test step is 0.014°, the scanning speed is 0.1s / step, and the scanning range is 5-40° (2θ). After testing, the X-ray powder diffraction sp...
Embodiment 2
[0033] Prescription: Dexoxiracetam (200g), 0.1M sodium dihydrogen phosphate (appropriate amount), calcium sodium edetate (300mg), aspartic acid (50g), mannitol (100g), water for injection to 1000ml. Preparation method: Take 800ml of water for injection, add the prescription amount of aspartic acid, mannitol and calcium sodium edetate, stir to dissolve, add the prescription amount of dexoxiracetam and stir to dissolve, and use 0.1M diphosphate Adjust the pH value of the sodium hydrogen solution to 5.2, add 0.1% activated carbon for needles according to the prepared amount, heat the liquid medicine to about 48°C, stir for 30 minutes, filter and decarbonize, add water for injection to the total amount, and then use a 0.22 μm microporous filter membrane Fine filtration, filling, half stoppering.
[0034] Freeze-drying process: divided into three stages: pre-freezing, sublimation drying and desorption drying. In the pre-freezing stage: the temperature of the shelf is reduced to -5...
Embodiment 3
[0036] Prescription: Dexoxiracetam (100g), appropriate amount of 0.1M sodium dihydrogen phosphate, disodium edetate (200mg), arginine (100g), and add water for injection to 1000ml.
[0037] Preparation method: Take 700ml of water for injection, add the prescribed amount of arginine and edetate disodium, stir to dissolve, add the prescribed amount of Dexoxiracetam and stir to dissolve, adjust with 0.1M sodium dihydrogen phosphate solution pH value is 6.0, add 0.2% activated carbon for injection according to the prepared amount, heat the liquid medicine to about 45°C, stir for 25 minutes, filter and decarbonize, add water for injection to the total amount, and then use a 0.22μm microporous filter membrane for fine filtration, pour Pack, half stoppered.
[0038] Freeze-drying process: Freeze-drying is divided into three stages: pre-freezing, sublimation drying and desorption drying. In the pre-freezing stage: the temperature of the shelf is reduced to -50°C, and the product is qu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com